Chemopreventive effect of dimethyl dicarboxylate biphenyl on malignant transformation of WB-F344 rat liver epithelial cells1
Introduction
Human hepatocellular carcinoma (HCC) is one of the most frequent malignant cancers. The carcinogenesis of HCC is a multifactorial event. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the most frequent causes of HCC[1]. Approximately 80% of human HCC are attributable to HBV infection[2]. Chronic HBV carriers are 100–400 times more likely to develop HCC than non-carriers[3]. HCV is the second most common cause of HCC after HBV[4]. Currently, HCC represents more than 4% of all cancer cases worldwide and causes at least 315 000 deaths every year[5]. Although early HCC can be cured by surgical resection, many HCC are asymptomatic, so most HCC patients are not diagnosed in time.
An effective approach to cancer control is chemo-prevention. It is known that the therapy of both chronic HBV and HCV generally involves a long-term course. An anti-hepatitis drug with an inhibiting or a suppressing effect on the development of hepatocarcinogenesis, besides its improvement of abnormal liver function, would be of great clinical value.
Dimethyl dicarboxylate biphenyl (DDB) is a synthetic analogue of Schizandrin C, which was isolated from Fructus Schizandrae chinensis[6]. Since 1983, DDB has been widely used to treat hepatitis B patients in China and is exported to Korea, Egypt, Vietnam, Indonesia, Pakistan, and Burma for the treatment of HBV and HCV. The results of the clinical application indicated that DDB markedly improved impaired liver functions, such as the elevated serum transaminase, bilirubin, α-fetal protein, and symptoms of the patients. Pharmacologically, DDB has a protective action against experimental liver injury in mice and rats[7,8,9]. DDB also had anticancer activity and differentiation-inducing effect on cancer cells[10]. In the present paper, the chemoprevention effect of DDB on hepatocellular carcinogenesis in vitro is studied.
Materials and methods
Chemicals DDB with 99% purity was provided by the Beijing Union Pharmaceutical Plant. As DDB is not water-soluble, it was dissolved in dimethyl sulfoxide (Me2SO) for in vitro use. 3-Methylcholanthrene (3MC), 12-O-tetradecanoyl phorbol 13-acetate (TPA), MTT, and Lucifer yellow CH were obtained from Sigma Chemical Company. Other chemicals were of analytical grade and purchased from Beijing Chemical Company.
Cell culture WB-F344 cells were grown in DMEM (GIBCO) media containing 10% newborn calf serum, 100 kU/L penicillin, and 100 mg/L streptomycin in a 37 °C humidified incubator containing 5% CO2 and 95% air, and passaged using 0.25% trypsin plus 0.02% EDTA treatment. The culture medium was changed every other day.
MTT assay Cytotoxicity was determined by MTT assay according to the method of Mosmann[11]. WB-F344 cells (3×103cells per well) were plated on 96-well plates, and 24 h later various concentrations of DDB were added (0.5 µmol/L–100 µmol/L). The cells were incubated at 37 °C in a CO2 incubator for 72 h. The culture supernatant was sucked out and MTT 0.5 g/L stock solution was added to each well. After 4 h of incubation, Me2SO was added. The optical density of each well was determined by a microplate reader at a wavelength of 570 nm. The values of absorbance were expressed as relative viable cell number.
In vitro transformation of WB-F344 cells WB-F344 cells were seeded on 25-cm2 tissue culture flasks containing the complete medium at a density of 4×103cells per flask. The medium was replaced with the complete medium containing 3MC (2 mg/L) or 0.1% Me2SO 24 h after seeding, and the cells were incubated for another 72 h. After the removal of the medium, the cells were washed twice with sterile phosphate-buffered saline (PBS) and incubated in fresh medium for 4 d. The cells were then incubated with medium containing 100 µg/L TPA. The TPA-containing medium was changed every 2–3 d for 14 d. After sucking out the TPA-containing medium, the cells were washed twice with sterile PBS and then incubated in fresh medium containing 10% newborn calf serum. The fresh medium was changed every 2 d until d 30. DDB was added to the medium from 24 h after cell seeding until the end of the experiment. At d 30, three of these flasks from each group were stained with Wright-Giemsa, and scored for transformed colonies. The remainders were used for soft-agar assay.
Soft-agar colony formation assay Cells derived from each group were seeded separately. Agar (0.6%) in the complete medium was kept at 44 °C and poured into 6-well plates (2 mL per well) as to form the lower layer. After the agar medium had set, 1×104cells per well in 2 mL of 0.3% agar (44 °C) were layered onto the gelled agar as the form of the upper layer. The cells were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. On d 9 and d 18, 1 mL of 0.3% agar in the complete medium was added. After 28 d, colonies of more than 20 cells were counted under contra-phase microscope.
Cell-cell communication assay The scrape loading/dye transfer (SL/DT) technique was used to detect GJIC according to the method of E1-Fouly et al[12]. WB-F344 cells were pretreated with various concentrations of DDB for 1 h at 24 h prior to the addition of TPA (100 µg/L) for 1 h. The other cells were pretreated with 4 µmol/L DDB for 24 h, 48 h, and 72 h before treatment with TPA. Following incubation, the cells were washed twice with PBS. Then Lucifer yellow CH (a fluorescent dye permeating gap-junctional channels) was added and several scrapes were made with a surgical steel-bladed scalpel at low light intensities. These scrapes were performed to ensure that the scrape traversed a large group of confluent cells. After 3 min incubation, the cells were washed with PBS again. Dye migration was observed and photographed with an inverted fluorescent microscope (Olympus, Japan) at ×200 magnification. The number of dyed cells represents the ability of cells to communicate via GJIC. GJIC data are reported as a percentage of the corresponding mean control value. The data are obtained from 3 views per plate, pooled 4 separate plates for each point.
Statistical analysis Results are expressed as mean±SD. To compare mean values between 2 groups, the Student’s t-test was used. P<0.05 was considered statistically signifi-cant.
Results
Cytotoxicity of DDB to WB-F344 cells To select the appropriate doses of DDB for the present study, the cytotoxicity of DDB to WB-F344 cells was assessed using the MTT assay. No significant cytotoxic effect on the cells was observed when the concentrations of DDB were below 4 µmol/L (Table 1). Therefore, 1 µmol/L, 2 µmol/L, and 4 µmol/L of DDB were used in the subsequent experiments.
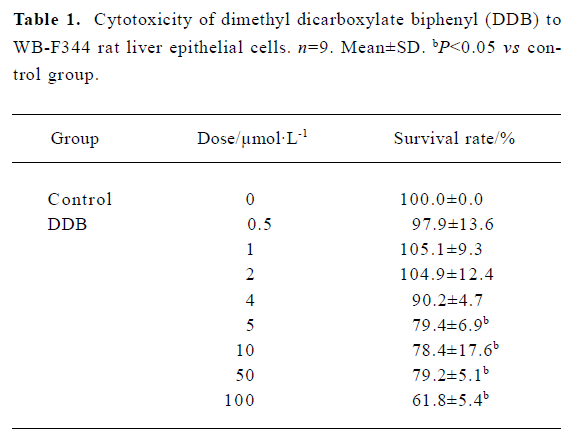
Full table
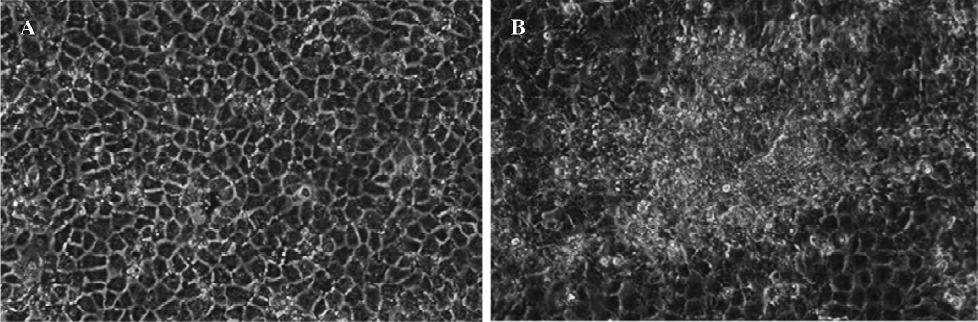
Effect of DDB on two-stage transformation of WB-F344 cells A two-stage (initiation and promotion) chemical induction oncogenesis model with WB-F344 cells was established. The WB-F344 cells became transformed after 3-MC (2 mg/L) initiation for 72 h and then TPA (100 µg/L) promotion for 14 d. The transformed cells were grown in a disorganized multilayer instead of in a monolayer (Figure 1). DDB at concentrations of 1 µmol/L, 2 µmol/L, and 4 µmol/L markedly inhibited transformation of WB-F344 cells in a dose-dependent manner. The average number of transformed foci decreased dramatically by 10.0%, 37.2%, and 47.4%, respec-tively, after DDB treatment (Table 2).
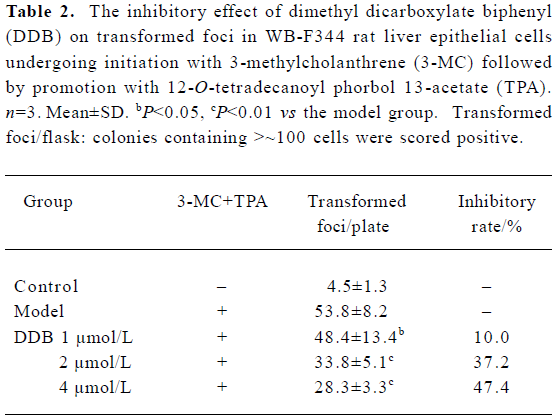
Full table
Effect of DDB on colony of transformed WB-F344 cells in soft agar To evaluate the tumorigenic potential of the treated WB-F344 cells, the efficiency of their soft-agar colony formation was determined. As shown in Table 3, no colony formed in soft agar in untreated WB-F344 cells, whereas the cells initiated with 3-MC and promoted with TPA developed the transformed phenotype of colony formation in soft agar. A remarkable increase in colony numbers was observed. The cells treated with 2 µmol/L and 4 µmol/L DDB also developed the transformed phenotype of colony formation, but the colony numbers significantly decreased compared with the model group.
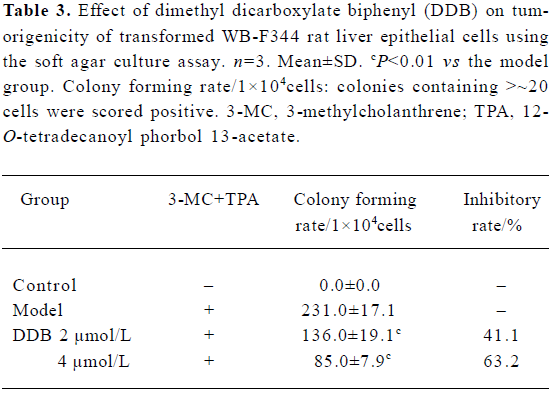
Full table
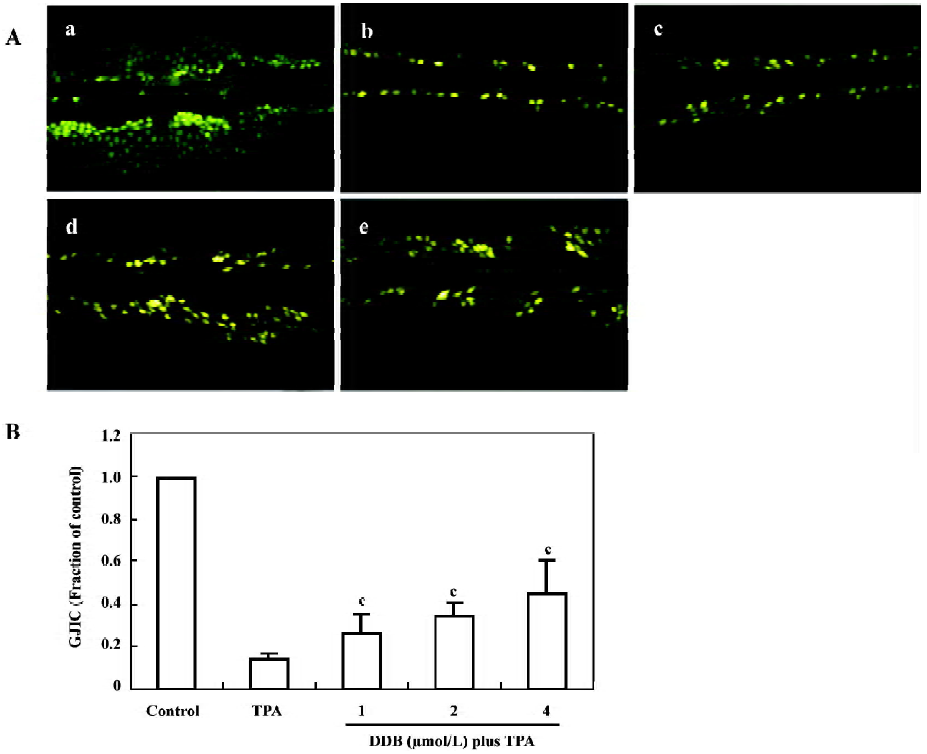
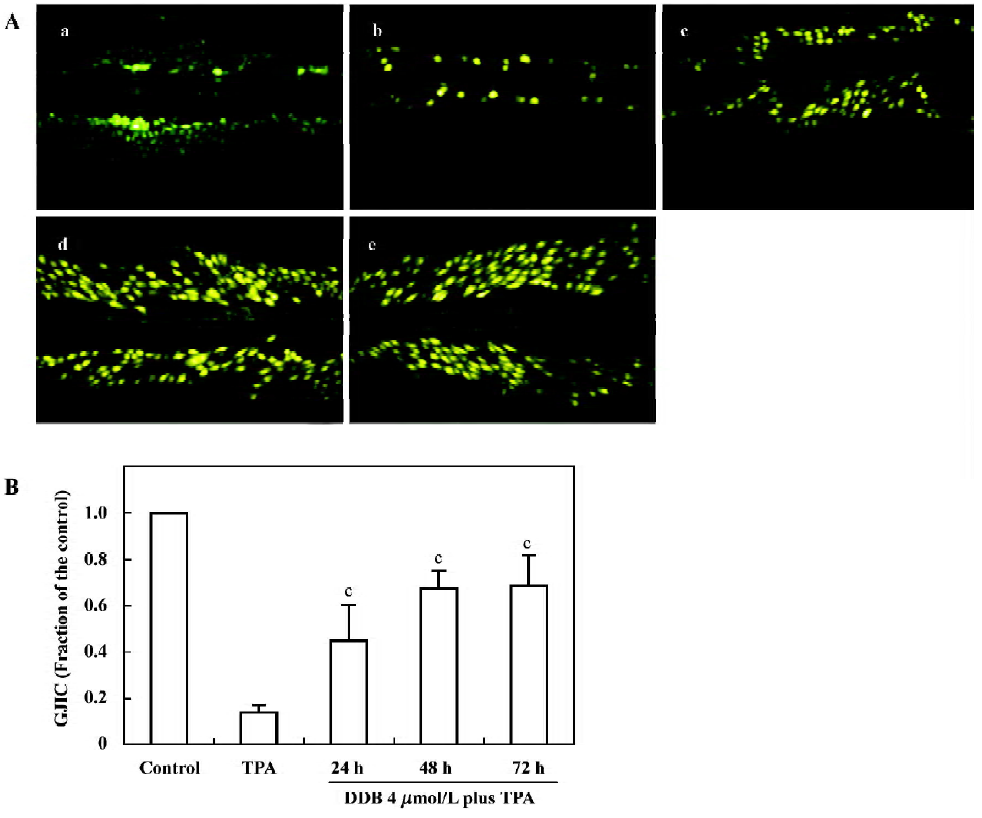
Effect of DDB on GJIC The GJIC of normal WB-F344 cells was well- characterized and did not decrease during the experimental incubation period (Figure 2Aa). After exposing the cells to TPA (100 µg/L) for 1 h, over 85% inhibition of GJIC was detected. The Lucifer yellow CH only stayed at the incision sites or artificially damaged cells (Figure 2Ab). When the cells were pretreated with DDB 1 µmol/L, 2 µmol/L, and 4 µmol/L, respectively, for 24 h, a dose-dependent inhibition of TPA-induced downregulation of GJIC was observed. The GJIC recovered to 25.6%, 34.6%, and 44.9% of the control group, respectively (Figure 2B). The time-dependent inhibitory effect of 4 µmol/L DDB on TPA-induced down-regulation of GJIC is shown in Figure 3. By the addition of 4 µmol/L DDB for 24 h, 48 h, and 72 h, TPA-induced downregulation of GJIC was markedly reversed in a time-dependent manner.
Discussion
WB-F344 cells have often been used in the study of hepatocarcinogenesis[13]. In the present study, we found that the anti-hepatitis drug DDB at non-toxic doses markedly prevented the transformation of WB-F344 cells induced by 3-MC and TPA in vitro, which expressed as significant decrease of the number of transformed foci and the malignant degree of transformed cells.
It is well known that carcinogenesis is a multistage and multimechanism process, involving the irreversible alteration of a stem cell (the initiation phase), followed by the clonal proliferation of the initiated stem cell (the promotion phase), from which the acquisition of the invasive and metastasis phenotypes are generated (the progression phase). Intervention to prevent cancer can occur at each step. For chemoprevention of carcinogenesis, the development of anti-tumor promoting agent has been regarded as the most effective pathway.
Intercellular communication is necessary in multicellular organisms to maintain tissue homeostasis and to control cell growth and differentiation. Gap junction channels play an important role in intercellular communication by providing a direct pathway for the movement of molecular information, including ions, polarized and non-polarized molecules up to a molecular mass of 1 kDa between adjacent cells[14,15]. Much evidence has been documented to support the hypothesis that the downregulation of GJIC is a cellular event underlying the tumor promotion process, and that any treatment to prevent downregulation of GJIC is important in prevention of tumor promotion[16,17]. Many tumor promoters have been shown to inhibit gap junctional communication in vitro[18,19]. TPA is a well-known classical inhibitor of cell communication in most cells, including the WB-F344 cell[20]. In the present study, the underlying mechanisms of DDB against hepatocarcinogenesis were investigated during the promotional phase using TPA to inhibit GJIC. The WB-F344 cells are known to have high GJIC. The treatment with TPA significantly inhibited GJIC, as was determined using the SL/DT assay. The counteracting effect of DDB on GJIC inhibition caused by TPA suggests that DDB has a significant action in maintaining GJIC function, and that it might be beneficial in preventing tumor promotion.
In summary, the results of the present study suggest that DDB can prevent the malignant transforming of WB-F344 cells induced by 3-MC and TPA in vitro. The restoration of GJIC in the promotion phase should contribute, at least in part, to the anti-hepatocarcinogenic property of DDB. We conducted other experiments and found that DDB significantly inhibited liver carcinogenesis induced by DEN/PB in mice; the data from these experiments will be published in another paper soon. Both in vitro and in vivo experiments demonstrated that DDB had a chemopreventive effect on hepatocarcinogenesis. It is worthy to pay attention to whether DDB potentially prevents liver carcinogenesis in patients with chronic viral hepatitis.
References
- Feitelson MA, Pan J, Lian Z. Early molecular and gentic determinants of primary liver malignancy. Surg Clin N Am 2004;84:339-54.
- Yerian LM, Anders RA, Tretiakova M, Hart J. Caveolin and thrombospondin expression during hepatocellular carcinogenesis. Am J Surg Pathol 2004;28:357-63.
- Birrer RB, Birrer D, Klavins JV. Review: Hepatocellular carcinoma and hepatitis virus. Ann Clin Lab Sci 2003;33:39-54.
- Robert S, Brown J, Paul JG. Scope of worldwide hepatitis C problem. Liver Transpl 2003;9:s10-s13.
- Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPARä activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochem Biophys Res Commun 2003;308:361-8.
- Liu GT. From the study of Fructus Schizandrae to the discovery of biphenyl dimethyl-dicarboxylate. Acta Pharm Sin 1983;18:714-20.
- Liu GT, Wang GF, Wei HL, Bao TT, Song ZY. A comparison of the protective actions of biphenyl dimethyl-dicarboxylate, trans-stilbene, alcoholic extracts of Fructus Schizandrae and ganoderma against expeimental liver injury in mice. Acta Pharm Sin 1979;14:598-604.
- Liu GT, Wei HL, Song ZY. Further studies on the protective action of biphenyl dimethyl-dicarboxylate (BDD) against experimental liver injury in mice. Acta Pharm Sin 1982;17:101-6.
- Wang GX, Ben CE, Ye BK, Yang M, Gao JL. Reparative effects of biphenyl dimethyl dicarboxylate on experimental liver injury in rats with histochemical and electronmicroscopy study. Chin J Integrat Tradit Western Med 1988;8:158-60.
- Liu ZY, Cui Q, Fu T, Liu GT. Inducing effect of dimethy-4,4'-dimethoxy-5,6,5',6-dimethylenedioxybipheny-2,2'-dicarboxylate (DDB) on differentiation of leukemia HL-60 cells. Natl Med J China 1996;76:214-7.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer: A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res 1987;168:422-30.
- Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of “oval” cells. Exp Cell Res 1984;154:38-52.
- Cho JH, Cho SD, Hu H, Kim SH, Lee SK, Lee YS, et al. The role of ERK1/2 and p38 MAP kinase in the preventive mechanisms of mushroom phellinus linteus against the inhibition of gap junctional communication by hydrogen peroxide. Carcinogenesis 2002;23:1163-9.
- Simon AM, Goodenough DA. Diverse functions of vertrbrate gap junction. Trends Cell Biol 1998;8:477-83.
- Niloofar A-A, Wilhelm S, Helmut S. (-)-Epicatechin effects in rat liver epithelial cells: stimulation of gap junctional communication and counteraction of its loss due to the tumor promoter 12-O-tetradecanoylthorbol-13-acetate. Biochem Pharm 2002;63:2145-9.
- Hu J, Engman L, Cotgreave IA. Redox-actine chalcogen-containing glutathione peroxidase minetics and antioxidants inhibit tumour promoter-induced downregulation of gap junctional intercellular communication between WB-F344 liver epithelial cells. Carcinogenesis 1995;16:1815-24.
- Guan X, Randall GR. Gap junction endocytosis and lysosomal degradation of connexin43-P2 in WB-F344 rat liver epithelial cells treated with DDT and lindane. Carcinogenesis 1996;17:1791-8.
- Ping R, Parmender PM, Randall JR. Inhibition of gap junctional intercellular communication by tumor promoters in connexin43 and connexin32-expressing liver cells: Cell specificity and role of protein kinase C. Carcinogenesis 1998;19:169-75.
- Kang KS, Kang BC, Lee BJ, Che JH, Li GX, Trosko JE, et al. Preventive effent of epicatechin and ginsenoside Rb2 on the inhibition of gap junctionsl intercellular communication by TPA and H2O2. Cancer Lett 2000;152:97-106.
