Roles of nitric oxide in protective effect of berberine in ethanol-induced gastric ulcer mice1
Introduction
Berberine, also known as huangliansu, is an isoquinoline alkaloid derived from the Chinese herb Huanglian, Huangbai and other plants. It has multiple pharmacological actions, such as antibacterial activity and anti-inflammatory effects, and is used in the treatment of diarrhea and other digestive disorders[1]. Berberine has been found to protect the gastric mucosa and inhibit gastric ulcers[2], but the pharmacological mechanisms for the protective effect of berberine are not clear. Accumulating evidence from both animal and human studies indicates that nitric oxide (NO) plays a key role in normal wound repair. The beneficial effects of NO on wound repair may be attributed to its functional influences on angiogenesis and inflammation[3]. A recent study demonstrated that NO generated from endothelial nitric oxide synthase (eNOS) played an important role in gastric ulcer formation and gastric healing[4]. However, NO generated from inducible nitric oxide synthase (iNOS) participates in ulcer formation through the production of peroxide free radicals and their cytotoxic action[5]. At the same time, it was reported that berberine could induce the thoracic aorta of rats to release endothelial NO[6], and 13-methylberberine and 13-ethylberberine reduced the production of NO and the expression of iNOS protein in a concentration-dependent manner in lipopolysaccharide (LPS)-stimulated macrophages[7]. We therefore inferred that it was possible for berberine to protect the gastric mucosa and accelerate the healing of peptic ulcers through the NO pathway. In the present study, we attempted to investigate whether the protective effect of berberine on gastric mucosa was related to NO and nitric oxide synthase (NOS).
Materials and methods
Drugs and reagents Berberine was supplied by Prof Jia-lin WANG (Department of Pharmacology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China). The NO kit was supplied by Nanjing Jian Cheng Bioengineering Company (Nanjing, China).
Animals The present study was carried out using 72 Kunming mice (Certificate N
Induction of gastric ulcer Gastric ulcers were produced by oral administration of ethanol 24 h after starvation. Each of the mice was given 0.1 mL ethanol (100%, anhydrous alcohol).
Drugs treatments and measurement of ulcer size Animals were divided into 3 groups of 24 mice each. Group I received saline at a dose of 0.1 mL/kg (ip) and served as the control group. Group II received berberine at a dose of 5mg/kg (ip) while group III received berberine at a dose of 50 mg/kg (ip).
Drugs were given 30 min before the oral administration of ethanol. The mice were killed by cervical dislocation 1 h, 2 h, 3 h and 6 h after oral administration of ethanol, and the gastric juice was sucked from the stomach before the stomach was removed. It was then opened along the greater curvature and the mucosa of the glandular portion of the stomach was rinsed gently with saline. Macroscopic damage was assessed and the number of ulcers, ulcer severity and ulcer index (UI) were recorded. Because each mouse had many lesions or ulcers and most gastric mucosal lesions were punctate or linear, each ulcer was graded according to severity using a scale of 1–4, as follows: 1, punctate ulcer; 2, linear ulcer of length ≤2 mm; 3, linear ulcer of length 2 mm–4 mm; and 4, linear ulcer of length ≥4 mm. The UI was calculated by summing the total number of ulcers, as described previously[8,9]. The gastric tissues were stored at -70 °C until biochemical analysis.
Measurement of nitric oxide The content of nitric oxide in the gastric juice and gastric tissue was measured through the method of nitric acid reductase, and the operational processes were carried out strictly in accordance with the NO kit instructions.
Reverse-transcription polymerase chain reaction for the detection of eNOS and iNOS mRNA The stomachs were removed from the saline-treated (0.1 mL/kg) and berberine-treated (50 mg/kg) groups both before and after ulcer induction (1 h, 3 h and 6 h) for the determination of eNOS and iNOS mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR) using specific primers. Total RNA was isolated from gastric tissues using Trizol reagent (Gibco BRL, Gathersburg, MD, USA). First-strand cDNA was synthesized from 5 µg of total cellular RNA using oligo-(dt)20 primers with the thermoscript RT-PCR system (Gibco BRL). PCR cycles were carried out for amplification of eNOS, iNOS and β-actin cDNA using a thermal cycler (Perkin-Elmer Corporation 850 Lincoln Centre Drive, Foster City, California, USA) and oligonucleotides (Boya, Shanghai, China). The primers for eNOS were 5'-TTC CGG CTG CCA CCT GAT CCT AA-3' (sense) and 5'-AAC ATA TGT CCT TGC TCA AGG CA-3' (antisense)[10]. The β-actin primer sequences for iNOS were 5'-CGG GCA TTG CTC CCT TCC GAA AT-3'(sense) and 5'-CTT CAT GAT AAC GTT TCT GGC TCT-3' (antisense)[11]. The oligonucleotide primer sequences for β-actin were 5'-TCA CCC ACA CTG TGC CCA TCT ACG A-3' (sense) and 5'-GGA TGC CAC AGG ATT CCA TAC CCA-3' (antisense). The number of PCR cycles was adjusted carefully to avoid saturation of the amplification system. In the RT step, the cellular mRNAs were reverse-transcribed into a “library” of cDNAs. This cDNA library was then used for the analysis of various genes in the PCR step. Briefly, total RNA (2.5 µg) from each of the tissues was reverse transcribed into single-stranded cDNA in a 20-µL reaction mixture containing: 4 µL 5' buffer, 20 IU ribonuclease inhibitor, 10 mmol/L dNTP, 0.5 µg oligo-(dt)20 primer, 20 IU RNasin and 200 IU M-MLV reverse transcriptase. After incubation for 1 h at 42 °C, the RT mixture was incubated at 70 °C for 10 min to inactivate the reverse transcriptase. PCR was then carried out using 5 µL cDNA in a final reaction volume of 50 μL. The assay mix contained 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), 1.5 mmol/L MgCl2, 0.2 mmol/L dNTP, 1 mmol/L of the respective primers and 1.5 IU of Taq DNA polymerase. The PCR cycling program was set for 1 cycle of pre-denaturation at 94 °C for 1 min, and then 35 cycles at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, followed by 1 cycle at 72 °C for 5 min. PCR products were visualized by UV illumination after electrophoresis on a 2% agarose gel containing 0.5 µL ethidium bromide. The location of a predicted product was confirmed using a 100 bp DNA ladder (Gibco BRL) as a standard size marker. The gel photographs were scanned with a computerized densitometer (SYNGENE, London, Britain). The signals for eNOS and iNOS mRNA were standardized against the β-actin signal for each sample and the results are expressed as eNOS and iNOS mRNA/β-actin mRNA ratio.
Statistical analysis All of the data were expressed as mean±SD and the analysis was carried out using the t-test. Values of P < 0.05 were considered statistically significant.
Results
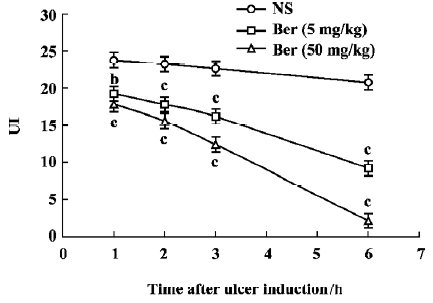
Effects of berberine on ethanol-induced gastric lesions The UI of ethanol-induced gastric lesions are shown in Figures 1 and 2. In the control group, the UI at 1 h, 2 h, 3 h and 6 h after oral administration of ethanol averaged 23.8±1.4, 23.3±2.2, 22.3±1.2 and 20.8±1.1, respectively. The UI in the berberine-treated groups (5 mg/kg and 50 mg/kg) were less than the control group and this effect was time-dependent (P< 0.05 and P<0.01, respectively).
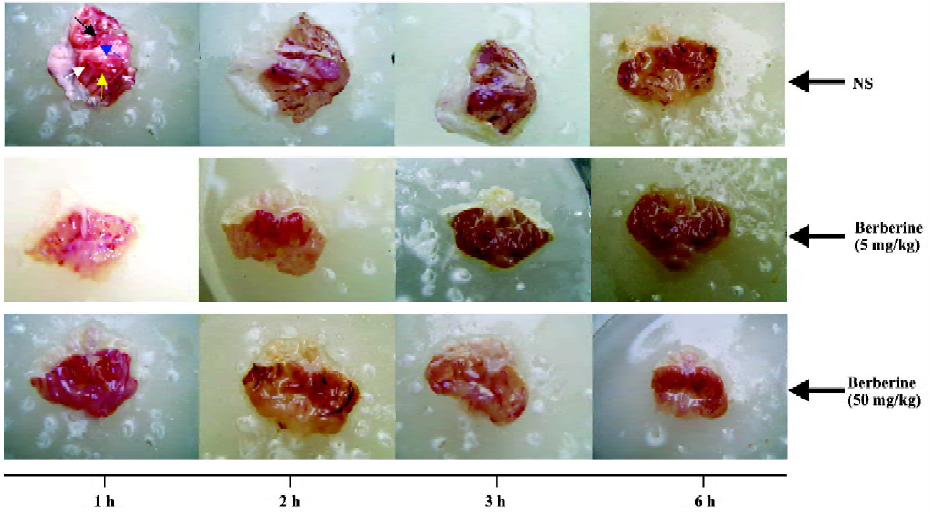
Change in nitric oxide content with the time The content of NO in gastric tissue and gastric juice is shown in Table 1. In the control group, the content of NO in gastric juice at 1 h, 2 h, 3 h and 6 h after oral administration of ethanol was 73.3 ±7.3 µL/L, 94.0±9.2 µL/L, 109.6±6.4 µL/L and 138.2±10.2 µL/L, respectively, while the NO content in gastric tissue averaged 5.8±1.1 µmol/g protein, 8.3±1.1 µmol/g protein, 9.8±1.1 µmol/g protein and 11.9±1.2 µmol/g protein, respectively. However, in both gastric tissue and gastric juice the content of NO in the berberine-treated groups (5 mg/kg and 50 mg/kg) was higher than that in the control group at 1 h after oral administration of ethanol (P<0.05, P<0.01), but lower at 6 h after oral administration of ethanol (P<0.05, P<0.01).
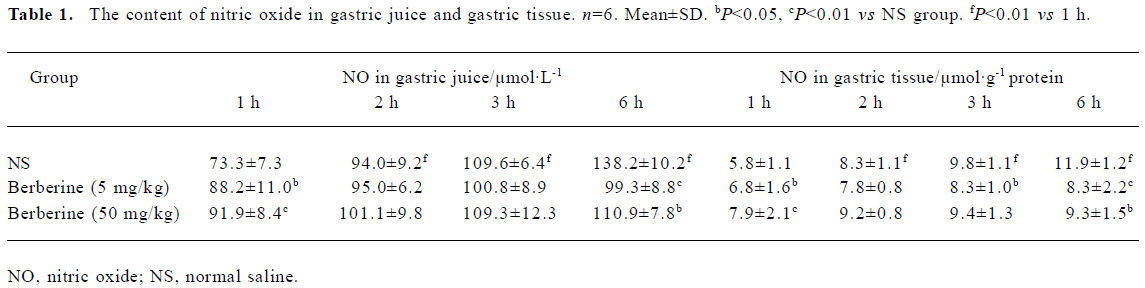
Full table
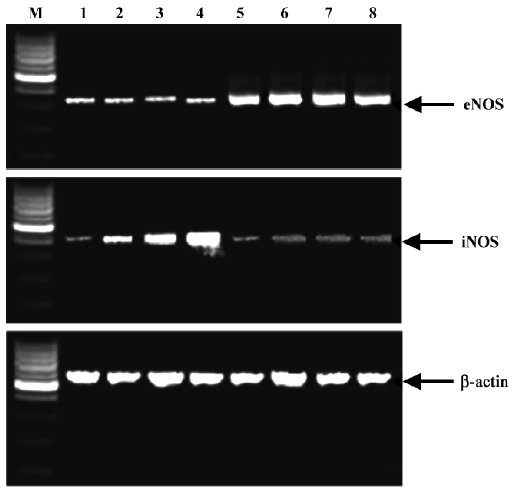
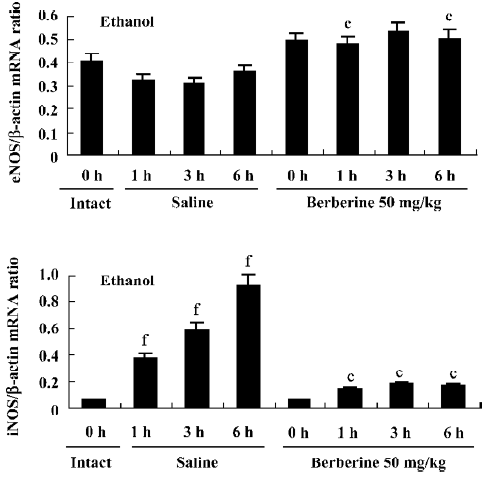
Expression of eNOS and iNOS mRNA Expression of iNOS and eNOS mRNA is shown in Figures 3 and 4. In the base statement the mRNA of eNOS could be expressed while that of iNOS could almost not be expressed. In the control group the expression of eNOS was inhibited and iNOS was enhanced by ethanol, while in the berberine-treated groups the expression of eNOS could be enhanced and iNOS could be inhibited.
Discussion
Nitric oxide plays an important role in the host defense and inflammatory response[12,13]. It also plays an important role in the mechanism of gastric mucosal protection and injury induced by pressure, ethanol, stress and endotoxins[14–17]. Endogenous NO has a dual action in the gastrointestinal tract: protective effects by constitutive nitric oxide synthase (cNOS)/NO and proulcerogenic effects by iNOS/NO[8].
cNOS lies in gastric endotheliocytes in the gastric tissue, also called eNOS[18]. NO derived from eNOS is a pivotal mediator to accelerate gastric ulcer healing; it maintains the integrity of the gastric epithelium, regulates gastric mucosal blood flow, and stimulates gastric mucus secretion and synthesis[19]. On the other hand, NO stimulates the release of vasoactive intestinal peptide (VIP) in gastric tissue and increases the concentration of cGMP[20]. NO/cGMP plays a central role in producing relaxation of mouse gastric fundus smooth muscle, and cGMP protects gastric parietal cells from ethanol-induced cytotoxicity, implicating a basolateral Cl- channel in the plasma membrane or that pepsinogen secretion stimulated by a Ca2+-mediated agonist is modulated by a NO/cGMP pathway[8]. The results of the present study have shown that the content of NO in tissue from the berberine-treated group was higher than control group at 1 h after oral administration of ethanol, and further study has shown that expression of eNOS is higher. Therefore, we might conclude that berberine can increase NO production through improving eNOS mRNA expression. The effect of berberine on NO formation in the vascular system was also demonstrated[6,21].
High expression of iNOS was found in ulcer tissue of the control group at 3 h and 6 h after ulcer induction. A similar phenomenon was also found by Peng et al[22]. Large amounts of NO synthesized from the inducible isoform have been implicated in tissue injury in the gut during inflammatory reactions[23]. Guo et al[24] also reported that high expression and activity of iNOS were found to coincide with severe inflammation in ulcer tissue. It is known that induction of high-output iNOS usually occurs in an oxidative environment, and thus high levels of NO have the opportunity to react with superoxide anion (O2–) leading to peroxynitrite (ONOO–) formation and cell toxicity, protein tyrosine nitration, hydroxyl radical production and tissue damage[24,25]. The results of the present study have shown that iNOS is under low-expression conditions at all times in the berberine-treated groups, as the content of NO had no peak and it was kept at a relatively steady level. This demonstrated that berberine might decrease NO production through inhibition of iNOS expression in the gastric ulcer tissue, prevent the abundant release of NO that aggravates gastric mucosal injury and, ultimately, improve the healing of ulcers. The study of Lee et al[7] also showed that 13-methyberine and 13-ethyberberine both inhibited iNOS expression in LPS-stimulated macrophages. In conclusion, the protective effect of berberine results in an increase in eNOS mRNA expression, which inhibits the iNOS mRNA expression process.
References
- Pan JF, Yu C, Zhu DY, Zhang H, Zeng JF, Jiang SH, et al. Identification of three sulfate-conjugated metabolites of berberine chloride in healthy volunteers’ urine after oral administration. Acta Pharmacol Sin 2002;23:77-82.
- Hicano H. Protective effects of Huanglian on gastric mucosa. Foreign Med Sci 1998;20:31.
- Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin 2005;26:259-64.
- Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol 2000;279:341-6.
- Cho CH. Current roles of nitric oxide in gastrointenstinal disorders. J Physiol Paris 2001;95:253-6.
- Kang DG, Sohn EJ, Kwon EK, Han JH, Oh H, Lee HS. Effects of berberine on angiotensin-converting enzyme and NO/cGMP system in vessels. Vascul Pharmacol 2002;39:281-6.
- Lee DU, Kang YJ, Park MK, Lee YS, Seo HG, Kim TS, et al. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci 2003;73:1401-12.
- Jimenez D, Martin MJ, Pozo D, Alarcon C, Md JE, Bruseghini L, et al. Mechanisms involved in protection afforded by L-arginine in ibuprofen-induced gastric damage: role of nitric oxide and prostaglandins. Dig Dis Sci 2002;47:44-53.
- Qureshi MA, Darzi A, Leahy AL, Bouchier-Hayes DJ. Transient reduction in gastric acid secretion following gastric mucosal laser irradiation. Surg Laparosc Endosc 1998;8:356-9.
- Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res 2002;942:23-30.
- Garhart CA, Heinzel FP, Czinn SJ, Nedrud JG. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect Immun 2003;71:910-21.
- Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol 2000;279:341-6.
- Ma JJ, Li SA. Nitric oxide and gastric ulcer. Prog Physiol Sci 1998;29:260-1.
- Nishida K, Ohta Y, Ishiguro I. Role of gastric mucosal constitutive and inducible nitric oxide synthases in the development of stress-induced gastric mucosal lesions in rats. Biochem Biophys Res Commun 1997;236:275-9.
- Qiu BS, Pfeiffer CJ, Cho CH. Effects of chronic nitric oxide synthase inhibition in cold-restraint and ethanol-induced gastric mucosal damage in rats. Digestion 1996;57:60-6.
- Tepperman BL, Soper BD. Nitric oxide synthase induction and cytoprotection of rat gastric mucosa from injury by ethanol. Can J Physiol Pharmacol 1994;72:1308-12.
- Zhang GF, Zhang MA, Chen YR, Wang L. The roles of endothelin and nitric oxide in gastric mucosa injuries in rats with endotoxemia. World Chin J Digestol 2000;8 Suppl 8:24.
- Fischer H, Becker JC, Boknik P, Huber V, Lüss H, Neumann J, et al. Expression of constitutive nitric oxide synthase in rat and human gastrointestinal tract. Biochim Biophys Acta 1999;1450:414-22.
- Li Y, Wang WP, Wang HY, Cho CH. Intragastric administration of heparin enhances gastric ulcer healing through a nitric oxide-dependent mechanism in rats. Eur J Pharmacol 2000;399:205-14.
- Yu XE, Luo QN. Protective effects of exogenous nitric oxide on acid-ethanol induced gastric ulcer in guinea-pig. World Chin J Digestol 2000;8:224.
- Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol 2000;399:187-96.
- Peng X, Feng JB, Wang SL, You ZY, Li A. Alterations of nitric oxide synthase and nitric oxide in gastric tissues of burned rats. World Chin J Digestol 1997;5:765-6.
- Barrachina MD, Panes J, Esplugues JV. Role of nitric oxide in gastrointestinal inflammatory and ulcerative diseases: perspective for drugs development. Curr Pharm Des 2001;7:31-48.
- Guo JS, Cho CH, Wang WH, Shen XZ, Cheng CL, Koo MW. Expression and activity patterns of three inducible enzymes in the healing of gastric ulcers in rats. Word J Gastroenterol 2003;9:1767-71.
- Ding HL, Zhu HF, Dong JW, Zhu WZ, Yang WW, Yang HT, et al. Inducible nitric oxide synthase contributes to intermittent hypoxia against ischemia/reperfusion injury. Acta Pharmacol Sin 2005;26:315-22.
