Histidine enhances carbamazepine action against seizures and improves spatial memory deficits induced by chronic transauricular kindling in rats1
Introduction
Epilepsy is frequently accompanied by impairments in various cognitive functions. More than 45% of epileptics have psychological or social problems with behavioral manifestations and are completely or partly disabled[1]. Although the causes of cognitive impairment in patients with epilepsy have not yet been completely elucidated, 3 factors are proposed: the underlying etiology of the epilepsy, the effects of seizures themselves, and the side-effects of antiepileptic drugs (AEDs) at therapeutic doses[2]. There is a need for drugs or adjuvants that can simultaneously suppress seizures and ameliorate the concomitant cognitive impairment.
Brain histamine levels might play an important role in the regulation of seizure susceptibility. Injection of histidine ip, a precursor of histamine, and metoprine, a histamine N-methyltransferase inhibitor, or icv injection of histamine, inhibited seizures induced by pentylenetetrazol, maximal electroshock (MES) or amygdaloid kindling in mice or rats[3–6], whereas α-fluoromethylhistidine, a selective and irreversible histidine decarboxylase inhibitor, increases the duration of clonic convulsions induced by MES in mice[3] and causes severe seizure development in pentylenetetrazol-induced kindling[7]. In contrast, the involvement of histamine in learning and memory processes has also been well documented. Histamine significantly ameliorates memory deficits induced by aging, dorsal hippocampal lesions, scopolamine and MK-801, as determined in rats using passive and active avoidance tasks and the 8-arm radial maze[8–12]. It is proposed that histamine and histaminergic compounds might be useful adjuncts for conventional AEDs, with dual advantages: one for enhancement of the anticonvulsant efficacy of AEDs, and the other for improvement of the memory deficits occurring with epilepsy.
The objectives of the present study were to further clarify whether histidine can both enhance the anticonvulsant efficacy of AEDs and ameliorate the spatial memory impairment induced by seizures. The chronic transauricular kindling procedure was used in rats as a new animal model of epilepsy, and an 8-arm (4 arms baited) radial maze paradigm was used to evaluate spatial memory and differentiate between short-term and long-term memory.
Materials and methods
Animals All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (220–270 g, Grade II, Certificate N
Radial-arm maze training The apparatus used is described in our previous reports[9,10,13]. The rats were familiarized with the radial maze once per day for 2 d prior to training. Food pellets (45 mg each, Bio-Serv) were scattered over the entire maze surface, and 3 or 4 rats were simultaneously placed in the maze and allowed to explore and take food freely for 10 min. After adaptation, all rats were trained with 1 trial per day. In each trial, only 4 arms (3, 5, 6, and 8) were baited, and the sequence was never changed throughout the experiment. One rat was placed on the center platform that was closed off by a door. After 15 s, the door was opened and the rat was allowed to make an arm choice to obtain food pellets until all 4 pellets had been eaten or 5 min had elapsed. The number of entries into unbaited arms was regarded as the total error (TE). The number of entries into never-baited arm was taken as reference memory error (RME), whereas re-entry into arms where the pellet had already been eaten was considered as a working memory error (WME). Rats continued training until reaching a criterion of less than 1 error per trial for 5 consecutive trials. Memory retrieval was tested in the same maze.
Chronic transauricular kindling After successful training in the radial maze, each rat in the experimental group was given 1 subconvulsive electrical stimulation daily via ear-clip electrodes (40 mA, 0.2 s, Hugo Saches type 221) until fully kindled. Animals in the sham group had the electrodes applied, but no current was delivered. Each rat was placed separately under a glass funnel, and the appearance of clonic-tonic seizures was recorded. When rats exhibited clonic-tonic seizures after each of 3 consecutive stimulations, they were regarded as fully kindled and used for the drug study. The endpoint of efficacy was taken as the inhibition of tonic hindlimb extension (HLE). The number of animals showing complete abolition of tonic HLE was expressed as percent protection.
Measurements of brain histamine and γ-aminobutyric acid (GABA) content
Sample preparation Rats were sacrificed by decapitation. The brain was quickly removed, placed on an ice-cold stainless steel plate, and dissected into the cortex, hippo-campus, brainstem and hypothalamus according to the methods of Glowinski and Iversen[14]. The brain tissues were stored at -80 ºC until assayed. The tissue was homogenized in 3% perchloric acid containing 5 mmol/L disodium EDTA and 5-hydro-Nω-methyltryptamine in a Polytron homogenizer (Kinematica) at the maximum setting for 20 s in an ice bath. The homogenate was centrifuged at 15 000×g at 4 ºC for 20 min. Then, the supernatant was removed and filtered with a 0.22 µm polyvinylidene difluoride membrane.
Chromatographic conditions Tissue samples were analyzed by high performance liquid chromatography (HPLC) combined with electrochemical detection using a technique developed in our laboratory for the simultaneous and sensitive analysis of histamine and GABA. The system consisted of a model 582 pump, a model 540 autosampler and a 4-channel CoulArray electrochemical detector. The HPLC was controlled and the data acquired and analyzed using CoulArray software. All of the above equipment was obtained from ESA. After reacting with the derivate o-phthalaldehyde, analytes were separated on a 3 μm, 3 mm×50 mm Capcell Pak MG C18 column from Shiseido. A 2-component gradient elution system was used, with component A of the mobile phase being 100 mmol/L Na2HPO4, 13% acetonitrile, and 22% methanol, pH 6.8, and component B being similar to A except with 5.6% acetonitrile and 9.4% methanol. The gradient elution profile was as follows: 0–3.5 min, isocratic 100% B; 3.5–20 min, linear ramp to 0% B; 20–22 min, isocratic 0% B; 22–23 min, linear ramp to 100% B; 23–30 min, isocratic 100% B. Flow rate was set to 0.75 mL/min. The first cell was set at +250 mV, whereas the second cell was set at +350 mV. All standards were obtained from Sigma. The retention times of GABA and histamine were 15.16 min and 18.36 min, respectively.
Drugs Histidine monohydrochloride and carbamazepine (CBZ) were obtained from Sigma. Histidine was dissolved in saline, and CBZ was suspended in a 1% solution of Tween 80. All drugs were injected ip in a volume of 1 mL/kg of body weight. Histidine was injected 1 h before and CBZ was injected 0.5 h before transauricular electrical stimulation or before the radial maze test. Drugs were given once a week. The same animals were used repeatedly, and they experienced all doses of each drug given in ascending order.
Statistical analysis Results were expressed as mean± standard error of the mean and analyzed by one-way analysis of variance followed by Dunnett’s t-test. For percentage incidence, the χ2-test with Fisher’s exact test was used. All statistical analyses were carried out using SPSS 11.5 for Windows. P<0.05 was considered as statistically significant.
Results
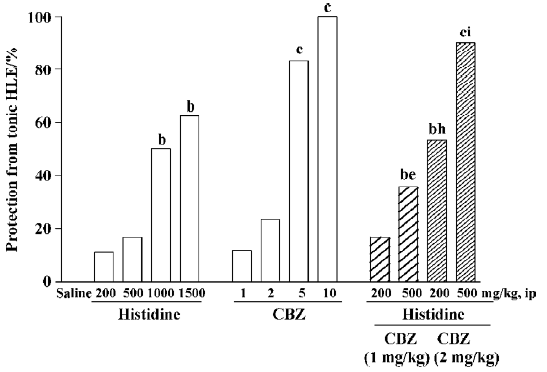
Effects of histidine and CBZ on transauricular kindled seizures in rats Both histidine and CBZ inhibited transauricular kindled seizures in a dose-dependent manner, as reflected by the increase in percentage protection against tonic HLE. Histidine at doses of 200 mg/kg and 500 mg/kg slightly inhibited tonic HLE, but had no significant effect, whereas at doses of 1000 mg/kg and 1500 mg/kg, it significantly inhibited tonic HLE (P<0.05). CBZ at doses of 1mg/kg and 2 mg/kg showed a tendency to inhibit tonic HLE, but no significant effect was observed, whereas at doses of 5 mg/kg and 10 mg/kg, it provided significant protection against tonic HLE (P<0.01). In addition, histidine at a low dose of 200 mg/kg showed a tendency to potentiate the anticonvulsant effects of 1 mg/kg CBZ and it significantly potentiated the anticonvulsant effects of 2 mg/kg CBZ (P<0.05). At a dose of 500 mg/kg histidine significantly potentiated the anticonvulsant effects of CBZ (1 mg/kg or 2 mg/kg) (P<0.05 and P<0.01, respectively) (Figure 1).
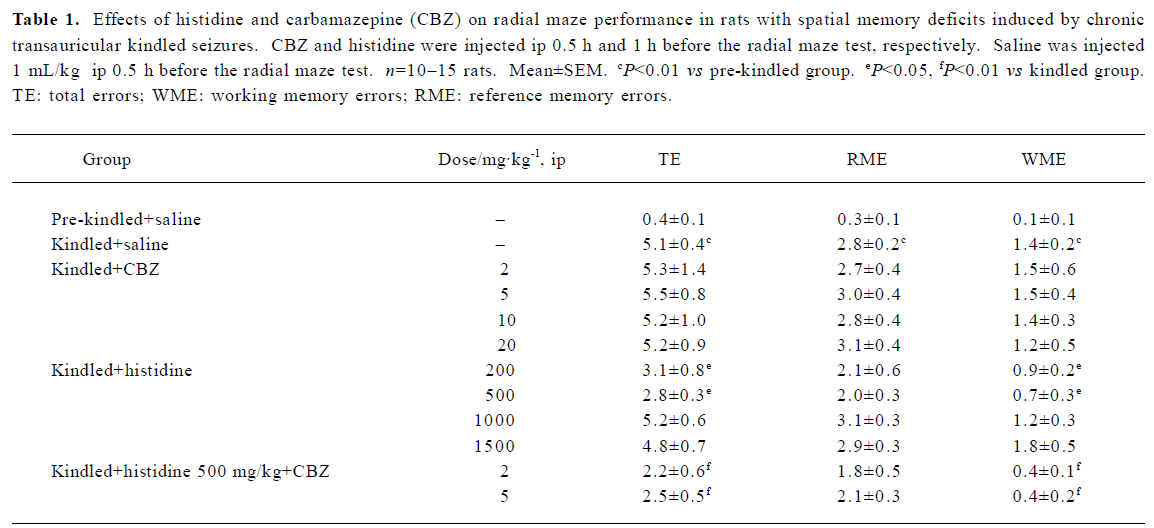
Effects of histidine and CBZ on radial maze performance in rats with spatial memory deficits induced by chronic transauricular kindled seizures The chronic transauricular kindling resulted in a significant increase in the number of TE, WME, and RME in the spatial memory retrieval process (P<0.01). Histidine at doses of 200 mg/kg and 500 mg/kg significantly decreased the number of TE and WME (P<0.05), but not the number of RME. However, histidine at doses of 1000 mg/kg and 1500 mg/kg had no ameliorative effect on the spatial memory deficits induced by transauricular kindled seizures. In contrast, CBZ (2, 5, 10, and 20 mg/kg) had no effect on memory impairment. The co-administration of CBZ also did not influence the improvement of spatial memory deficits induced by 500 mg/kg histidine (Table 1).
Full table
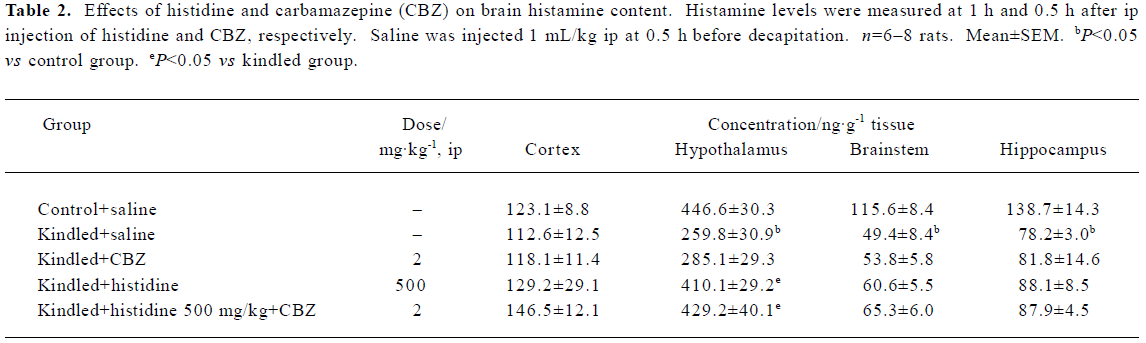
Effects of histidine and CBZ on brain histamine content Compared with control rats, chronic transauricular kindling produced a significant decrease in the histamine content of the hypothalamus (41.8%), hippocampus (43.6%), and brainstem (57.3%) (P<0.05). Histidine (500 mg/kg, ip) significantly increased the histamine content of the hypothalamus (157.9%) (P<0.05) and slightly increased that of the cortex (114.7%), hippocampus (112.7%) and brainstem (122.7%). CBZ (2 mg/kg) had no effect on the increased histamine content induced by histidine treatment (Table 2).
Full table
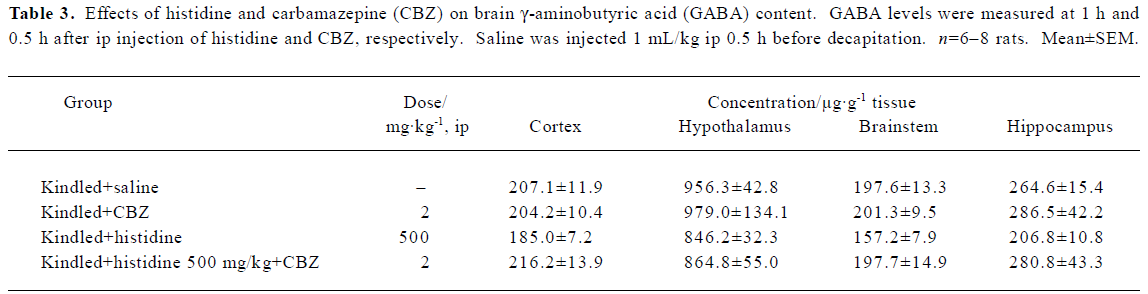
Effect of histidine and CBZ on brain GABA content Neither treatment with histidine (500 mg/kg) or CBZ (2mg/kg) alone, nor co-administration of the 2 drugs had any effect on brain GABA levels (Table 3).
Full table
Discussion
In the present study, we provide evidence for the first time that chronic transauricular kindling can induce not only generalized tonic-clonic seizures but also spatial memory deficits in rats. So far, few animal models are suitable for studing the memory deficits associated with epilepsy, especially for the form with generalized tonic-clonic seizures[15]. MES is a representative animal model of generalized tonic-clonic seizures[16]; however, it has no appreciable effect on cognitive behaviors, such as in passive avoidance learning[17,18]. This might be because MES is a model of acute seizures (reactive or provoked), rather than a model of epilepsy[19]. In the present study, we developed a chronic transauricular kindling model that was relatively simple and required neither expensive equipment nor highly skilled personnel. We found that chronic transauricular kindled seizures produced spatial memory deficits, which remained in a steady state for approximately 3 weeks after fully kindled (data not shown). Therefore, it is likely that the chronic transauricular kindling model is a very useful animal model for evaluating memory deficits associated with epilepsy, and in drug screening for both anticonvulsant and memory-improving actions.
In the present study, high doses of histidine (1000 mg/kg and 1500 mg/kg), a precursor of histamine, significantly inhibited chronic transauricular kindled seizures, but had no ameliorative effect on the spatial memory deficits induced by these seizures in rats. However, at doses of 200 mg/kg and 500 mg/kg, although it had no appreciable anticonvulsant effect, histidine significantly ameliorated spatial memory deficits. Therefore, it is proposed that at a lower dose, histidine combined with CBZ can simultaneously enhance the anticonvulsant effects and ameliorate spatial memory impairment in rats. Interestingly, we found that the lower doses of histidine significantly potentiated the protective effects of CBZ against transauricular kindled seizures and the cognitive improvement was not affected by co-administration of CBZ. Therefore, our results indicate that histidine, at a specific dosage range, potentiates both the anticonvulsant efficacy of CBZ and ameliorates the spatial memory deficits induced by chronic transauricular kindled seizures in rats. This indicates that histidine might serve as a beneficial adjuvant for the clinical treatment of epilepsy.
In addition, we found that chronic transauricular kindling resulted in a marked decrease of histamine content in the hypothalamus (41.8%), hippocampus (43.6%) and brainstem (57.3%). Consistent with our findings, decreased histamine content of the amygdala and hypothalamus has been reported to follow amygdaloid kindling[20]. The observed decrease in histamine levels following chronic transauricular kindled seizures further supports the concept of histamine as an endogenous anticonvulsant[21]. However, in contrast to our data, Vohora et al reported that acute MES significantly increased histamine concentration in the brainstem[22]. We have no data to explain this difference, which might be a result of different mechanisms underlying the seizures (acute vs chronic). Therefore, our results might at least partly elucidate why acute MES has no appreciable effect on cognitive behavior, whereas chronic transauricular kindling induces significant spatial memory deficits in rats[17,18].
It remains unclear why and how histidine potentiates the anticonvulsant effect of CBZ. A possible pharmacokinetic interaction is unlikely, because the plasma levels of CBZ remain unchanged in the presence of histidine[17]. Further-more, we found the GABA levels in the brain after treatment with histidine and/or CBZ were not significantly different from the values in the control group, which suggests that the enhancement of the anticonvulsant effect of CBZ at the doses used is independent of GABA levels. However, treatment with histidine (200 mg/kg or 500 mg/kg) significantly increased brain histamine content in the hypothalamus. The significant increase of brain histamine content induced by injection of histidine might at least partly contribute to the observed enhanced anticonvulsant efficacy of CBZ.
It is interesting that the effects of histidine on performance in the radial maze showed a bell-shaped inhibition. These results are in accordance with the reports by Sakai et al[23] and Ghi et al[24], who found dose-dependent biphasic effects of histamine on locomotor activity. Alvarez et al also reported that treatment with a lower dose of histamine (45 nmol) in the hippocampus improved memory retrieval in contrast to the effect of a higher dose (90 mg/kg)[25]. These findings suggest that the biphasic effects of histamine in the brain might be mediated through different mechanisms, and the memory improvement induced by histidine only occurs within a specific lower range of dosage.
We were further interested to find that histidine significantly decreased both the number of TE and WME, but not the number of RME induced by transauricular kindled seizures in rats. Our data suggests that histidine can only ameliorate the short-term memory deficit induced by the kindled seizures. Similar findings show that histidine, at doses that cause a significant increase of brain histamine content, improves the working memory deficit induced by 8-OH-DPAT and 7-chlorokynurenic acid in rats[26,27]. Our previous work also shows that the H3-antagonist clobenpropit facilitates deficits of working memory induced by intrahippocampal application of MK-801, and we demonstrate that the effects on working memory are a result of an increase in endogenous histamine, whereas the effects on reference memory are most likely a result of neurotransmitters other than histamine[28]. From the above evidence, it is likely that different mechanisms underlie working memory and reference memory, and brain histamine mainly participates in the former. Because short-term memory complaints are frequent in patients with epilepsy[29], the improvement in working memory induced by co-treatment with CBZ and histidine appears potentially useful.
In conclusion, we found that chronic transauricular kindling in rats was a very useful animal model for evaluating memory deficits associated with epilepsy. Histidine at relatively low doses significantly enhanced the anticonvulsant efficacy of CBZ against the kindled seizures and also had a significant ameliorative effect on the spatial memory impairment induced by these seizures. It is proposed that histidine, at a specific range of dosages, might serve as a beneficial adjuvant for the clinical treatment of epilepsy, especially when it is accompanied by impairment of spatial memory.
Acknowledgement
We are very grateful to Dr Iain C BRUCE (University of Hong Kong) for reading the manuscript.
References
- Genkova-Papazova MG, Petkova B, Shishkova N, Lazarova-Bakarova M. Effect of the calcium channel blockers nifedipine and diltiazem on pentylenetetrazole kindling-provoked amnesia in rats. Eur Neuropsychopharmacol 2001;11:91-6.
- Kwan P, Brodie MJ. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet 2001;357:216-22.
- Yokoyama H, Onodera K, Maeyama K, Yanai K, Iinuma K, Tuomisto L, et al. Histamine levels and clonic convulsions of electrically-induced seizure in mice: the effects of alpha-fluoromethylhistidine and metoprine. Naunyn-Schmiedeberg’s Arch Pharmacol 1992;346:40-5.
- Chen Z, Li WD, Zhu LJ, Shen YJ, Wei EQ. Effects of histidine, a precursor of histamine, on pentylenetetrazole-induced seizures in rats. Acta Pharmacol Sin 2002;23:361-6.
- Zhang LS, Chen Z, Ren KM, Leurs R, Chen JC, Zhang WB, et al. Effects of clobenpropit on pentylenetetrazole-kindled seizures in rats. Eur J Pharmacol 2003;482:169-75.
- Chen Z, Sakurai E, Hu WW, Jin CL, Kiso Y, Kato M, et al. Pharmacological effects of carcinine on histaminergic neurons in the brain. Br J Pharmacol 2004;143:573-80.
- Zhang LS, Chen Z, Huang YW, Hu WW, Wei EQ, Yanai K. Effects of endogenous histamine on seizure development of pentylenetetrazole-induced kindling in rats. Pharmacology 2003;69:27-32.
- Kamei C, Chen Z, Nakamura S, Sugimoto Y. Effects of intra-cerebroventricular injection of histamine on memory deficits induced by hippocampal lesions in rats. Methods Find Exp Clin Pharmacol 1997;19:253-9.
- Chen Z, Kamei C. Facilitating effects of histamine on spatial memory deficit induced by scopolamine in rats. Acta Pharmacol Sin 2000;21:814-8.
- Chen Z. Effect of histamine H3-receptor antagonist clobenpropit on spatial memory of radial maze performance in rats. Acta Pharmacol Sin 2000;21:905-10.
- Chen Z, Sugimoto Y, Kamei C. Effects of intracerebroventricular injection of α-fluoromethylhistidine on radial maze performance in rats. Pharmacol Biochem Behav 1999;64:513-8.
- Huang YW, Chen Z, Hu WW, Zhang LS. Facilitating effect of histamine on spatial memory deficits induced by dizocilpine as evaluated by 8-arm radial maze in SD rats. Acta Pharmacol Sin 2003;24:1270-6.
- Chen Z, Zhao Q, Sugimoto Y, Fujii Y, Kamei C. Effects of histamine on MK-801-induced memory deficits in radial maze performance in rats. Brain Res 1999;839:186-9.
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 1966;13:655-69.
- Majak K, Pitkanen A. Do seizures cause irreversible cognitive damage? Evidence from animal studies. Epilepsy Behav 2004;5 Suppl 11:35-44.
- Loscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res 1991;8:79-94.
- Kaminski RM, Zolkowska D, Kozicka M, Kleinrok Z, Czuczwar SJ. L-histidine is a beneficial adjuvant for antiepileptic drugs against maximal electroshock-induced seizures in mice. Amino Acids 2004;26:85-9.
- Swiader M, Wielosz M, Czuczwar SJ. Interaction of astemizole, an H1 receptor antagonist, with conventional antiepileptic drugs in mice. Pharmacol Biochem Behav 2003;76:169-78.
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 2002;50:105-23.
- Kamei C, Ishizawa K, Kakinoki H, Fakunaga M. Histaminergic mechanisms in amygdaloid-kindled seizures in rats. Epilepsy Res 1998;30:187-94.
- Leurs R, Blandina P, Tedford C, Timmerman H. Therapeutic potential of histamine H3 receptor agonists and antagonists. Trends Pharmacol Sci 1998;19:177-83.
- Vohora D, Pal SN, Pillai KK. Histamine and selective H3-receptor ligands: a possible role in the mechanism and management of epilepsy. Pharmacol Biochem Behav 2001;68:735-41.
- Sakai N, Sakurai E, Onodera K, Sakurai E, Asada H, Miura Y, et al. Long-term depletion of brain histamine induced by alpha-fluoromethylhistidine increases feeding-associated locomotor activity in mice with a modulation of brain amino acid levels. Behav Brain Res 1995;72:83-8.
- Ghi P, di Carlo G, Molinengo L. Effects of thioperamide on locomotor activity and on memory processes. Prog Neuro-psychopharmacol Biol Psychiatry 1998;22:387-95.
- Alvarez EO, Banzan AM. Effects of localized histamine microinjections into the hippocampal formation on the retrieval of a one-way active avoidance response in rats. J Neural Transm Gen Sect 1995;101:201-11.
- Isayama S, Sugimoto Y, Nishiga M, Kamei C. Effects of histidine on working memory deficits induced by the 5-HT1A-receptor agonist 8-OH-DPAT. Jpn J Pharmacol 2001;86:451-3.
- Nishiga M, Kamei C. Ameliorative effects of histamine on 7-chlorokynurenic acid-induced spatial memory deficits in rats. Psychopharmacology (Berl) 2003;166:360-5.
- Huang YW, Hu WW, Chen Z, Zhang LS, Shen HQ, Timmerman H, et al. Effect of the histamine H3-antagonist clobenpropit on spatial memory deficits induced by MK-801 as evaluated by radial maze in Sprague-Dawley rats. Behav Brain Res 2004;151:287-93.
- Shannon HE, Love PL. Effects of antiepileptic drugs on working memory as assessed by spatial alternation performance in rats. Epilepsy Behav 2004;5:857-65.
