Pharmacokinetics of sifuvirtide, a novel anti-HIV-1 peptide, in monkeys and its inhibitory concentration in vitro1
Introduction
Human immunodeficiency virus (HIV) infection is now a major public health problem for both developing and developed countries as it has dramatically increased the global burden of disease[1-5]. Many efforts have been made on research and discovery of anti-HIV drugs; however, it still remains difficult to develop more effective, affordable, and less toxic anti-HIV drugs for the increasing number of HIV-infected patients[6].
In recent years, more and more researchers have fixed their eyes on preventing the entry of HIV into a host cell at the step of gp41-mediated viral and cellular membrane fusion, which was denoted as fusion inhibitors[7]. The Food and Drug Administration of the United States announced, in 2003, the accelerated approval of enfuvirtide, the first fusion inhibitor, for use in combination with other anti-HIV medications to treat advanced HIV-1 infection in adults and children aged 6 years and older[8]. Enfuvirtide is administered twice daily by subcutaneous injection into the abdomen, the upper arm, or the anterior thigh for clinical therapeutics[9].
Sifuvirtide is a linear 36-amino acid peptide with an amino acid sequence of Acetyl-SWETWEREIENYTRQIYRILEE-SQEQQDRNERDLLE. It is a novel antiviral peptide that has been classified as a fusion inhibitor. The profiles of absorption, distribution, metabolism and excretion of sifuvirtide have been characterized in rats and monkeys by using radioactive 125I-sifuvirtide combined with radioactivity detection of samples derived from trichloroacetic acid precipitation or size-exclusion chromatography. For the requirement of clinical pharmacokinetic (PK) study, we have developed an advanced and validated on-line solid-phase extraction procedure combined with liquid chromatography tandem mass spectrometry (SPE-LC/MS/MS) approach for determination of non-labeled sifuvirtide in monkey plasma[10].
In the present study, an in vitro fusion inhibitory experiment was used to determine the 50% inhibitory concentration (IC50) of sifuvirtide. According to the results of 50 times lower IC50 value than enfuvirtide and the pharmacological experiments in monkeys, a relatively low clinical dosage was set at about 10 mg per adult, per day (while that of enfuvirtide was 180 mg[9]). The PK profiles and bioavailability of sifuvirtide following single sc or iv dosage of 1.2 mg/kg in rhesus monkeys were investigated. The preliminary results indicated that sifuvirtide made a difference in antiviral activity in vitro and PK behaviors in vivo compared with enfuvirtide.
Materials and methods
Drugs and reagents The sifuvirtide standard was provided by FusoGen Pharmaceuticals (Tianjin, China). Enfuvirtide was obtained from Hoffmann-La Roche (Nutley, NJ, USA). RPMI-1640 complete culture medium containing 10% heat-inactivated newborn calf serum (NCS), L-glutamine 2 mmol/L, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10 mmol/L, 2-mercaptoethanol 50 µmol/L, benzylpenicillin 100 kIU, and streptomycin sulfate 100 mg/mL were purchased from Gibco BRL (Gaithersburg, Maryland, USA) . C8166 and HIV-1IIIB chronically infected H9 cell lines (HIV-1IIIB/H9) were kindly donated by the Medical Research Council of United Kingdoms (AIDS Reagent Project) (London, UK). Drug-free monkey plasma was collected from 6 healthy rhesus monkeys of both sexes. Acetonitrile (ACN) and other solvents were of HPLC-grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). A protease inhibitor cocktail tablet, Complete Mini (Roche Diagnostics, Mannheim, Germany), without ethylene diamine tetraacetic acid, was used to prevent peptide degradation in biological matrixes. Formic acid (FA), trifluoroacetic acid (TFA) and sodium iodine were obtained from Aldrich Chemical Co (Milwaukee, WI, USA). Highly purified water was produced by a Millipore Simplicity 185 Unit (Millipore, Bedford, MA, USA). Mobile phase A (MPA) was 0.1% (v/v) FA in water. Mobile phase B (MPB) was 0.1% (v/v) FA in ACN.
Synthesis of the internal standard In this study, a novel strategy, non-radioactive iodination of peptides, was used to acquire an internal standard (IS) for the quantitative assay. Reaction of iodination was carried out mainly as described previously[11]. To acquire the highest productivity of peptide iodide, the molar ratio of iodine-127 to peptide standard was at least 20:1. The reaction was carried out at room temperature for 10 min. The reaction mixture was then desalted and purified by a RP-HPLC method utilizing a Vydac analytical C18 column (5 µm, 300 Å, 4.6 mm×150 mm; Vydac, Hesperia, CA, USA) with a gradient of MPB increasing from 25% to 75% in 20 min, coupled with fraction collection equipment (Pharmacia, Uppsala, Sweden). The purified product was stored at 4°C and identified using LC/MS/MS to be the goal peptide (purity >97%) with the 4-127I modifications (127I4-sifuvirtide) on tyrosine (Y) residues [SWETWEREIENY(I2)TRQIY(I2)RILEESQEQQDRNERDLLE].
Cell fusion assay The inhibition of sifuvirtide on cell-to-cell transmission of HIV-1 was determined by co-cultivation of uninfected normal cells and HIV-1IIIB chronically infected cells, as described previously[12]. Briefly, 3×104 C8166 cells were mixed with 1×104 HIV-1IIIB chronically infected H9 cells in 96-well plates in the presence or absence of sifuvirtide at various concentrations. Enfuvirtide was used as the control. After co-culture for 24 h at 37 °C in 5% CO2, the number of syncytia was counted using an inverted microscope. The percentage inhibition of cell fusion was calculated by dividing the number of syncytial cells in compound-treated cultures by those in untreated cultures. The IC50 was determined from a dose-response curve.
On-line sample preparation The on-line extraction column was prepared using an empty stainless steel column (size of 50 mm length×2.1 mm ID, 2 µm frit; GEA Co, Beijing, China) packed with reversed-phase materials. The packing materials used for SPE were obtained from Agilent (Zorbax C18; spherical; average particle size 50 µm; pore size 80 Å; Agilent; Waldbronn, Germany). Aliquots of calibrators or unknown samples (100 µL) were introduced onto the extraction column for on-line preparation and LC/MS/MS analysis. The plasma samples were diluted 5 times on-line, based on the design of an on-line splitter, between the sample loop and bypassing branch at a ratio of approximately 1:4. The whole process, including sample loading, extraction, separation and analysis, was carried out based on the column-switching program. Programmed events corresponding to valve switching were set up using the ChemStation (version 9.02) for Agilent 1100 series HPLC system and XcaliburTM (version 1.4) for LTQ-MS system.
LC/MS/MS system The separation and detection section, the LC/MS/MS system used for the assay, was made up of the Agilent 1100 series HPLC system (including an on-line degasser), a 100-vial autosampler, a temperature control compartment equipped with a 6-port/2-position switching valve, and a binary solvent delivery pump, coupled to a novel linear ion trap mass spectrometer, LTQ-MS (Thermo Finnigan, San Jose, CA, USA), equipped with a new atmospheric pressure ionization interface, Ion MAX ™. MS/MS detection was operated in electrospray ionization positive ion mode. The representative parameters of MS were as shown below. The spray voltage was 4.5 kV and the temperature of the heated capillary was set at 200°C. The flow rates of sheath gas, auxiliary gas and sweep gas were set (in arbitrary units/min) to 20, 5, and 4, respectively. Other parameters were optimized automatically by infusing the analyte in water:ACN:formic acid (49.9:50:0.1, v:v:v) at a flow rate of 200 µL/min. Quantitation was carried out using selected reaction monitoring (SRM) of the transitions m/z 946.5→m/z 871.8 for sifuvirtide and m/z 1047.2→m/z 972.3 for the IS (127I4-sifuvirtide).
Preparation of stock solutions and calibration standard samples Stock solutions of sifuvirtide were prepared at concentrations of 1.0 g/L in a MPA:MPB (90:10, v/v) solvent system. The drug-free monkey blood was deactivated by spiking with protease inhibitor cocktail tablets (2 tablets/5 mL whole blood). All blood samples collected from monkey sources were transferred immediately into tubes coated with calculated heparin and an inhibitor mixture of protease and peptidase on the tube surface. After incubation on ice for 30 min, they were centrifuged at 1000×g for 10 min. The supernatant of each was then harvested and stored at -80°C until analysis. The deactivated drug-free plasma from monkey sources was used as the standard dilution for the calibration samples. Each calibration curve consisted of blank samples, zero samples (plasma sample processed with IS), and 6 non-zero samples, ranging from 4.88 µg/L to 5000 µg/L. Before the assay, the frozen samples were thawed at ambient temperature, vortex-mixed and centrifuged at 1000×g for 5 min. For each of the calibrators or unknown samples, 100µL aliquots of supernatant were added to vials containing 10µL 127I4-sifuvirtide working solution (5 mg/L) and 10µL 2.5% ammonia solution (NH3·H 2O, w/v). The vials were vortex-mixed for 30 s, placed immediately in the autosampler tray and kept at room temperature for assay.
Method validation The calibration curve and the concentration of unknown sample were acquired using LCQuan software (version 2.0) from Thermo Finnigan. A weighting of 1/x2 (where x is the concentration of a given standard) was used for curve fit. The intra-day and inter-day accuracy and precision of the method presented here was investigated by analyzing plasma samples at the whole calibration concentration levels. The regression equation for the calibration curve was used to back-calculate the measured concentration for each standard, and the results were compared to the theoretical concentration to calculate the accuracy, expressed as a percentage of the theoretical value, for each standard measured.
Animal experiments Three rhesus monkeys (2♀ and 1♂, body weight of 4.1?0.4 kg) were provided by the Animal Center of the Academy of Military Medical Sciences, with certificate number 97083. The monkeys were fasted overnight prior to dosing and given free access to food and water 48 h post dosage. A dosing solution (1.0 g/L) was formulated in saline. Sifuvirtide was administered iv to monkeys at 1.2 mg/kg via a side femoral venous catheter. Blood samples (0.5 mL) were collected from a femoral venous catheter on the opposite side at 0 min, 1 min, 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, and 48 h after dosing. After a 7-d washout period, the monkeys were administered the same dosage and blood samples were harvested at 0 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, 5 h, 6 h, 8 h, 10 h, 12 h, 24 h, 36 h, and 48 h following dosing. All samples were harvested and treated in the same way as calibrators. A 200 µL aliquot from each time point was used for the assay. The plasma samples from 1 min, 5 min, and 15 min after iv were diluted 5 times because their concentrations were suspected to be higher than detectable levels. The curve according to plasma sifuvirtide concentration versus time after dosing was obtained using Origin software (Version 5.0; Microcal, Northampton, MA, USA).
Estimation of pharmacokinetic parameters Pharmacokinetic modeling and estimation of PK parameters were carried out using Excel XP software (Microsoft, Redmond, WA, USA). Cmax and Tmax were observed values. Other PK parameters, including area under the concentration-time curve (AUC), apparent plasma terminal elimination T1/2, mean residence time (MRT), volume of distribution (Vd) and systematic clearance (CL), were calculated according to a previous description of the non-compartmental approach[13]. In addition, the absolute bioavailability (Fabs, %) was calculated by the following equation: [(subcutaneous AUC0-t/intravenous AUC0-t) ×100%].
Results
Effects of sifuvirtide on HIV-1 entry Sifuvirtide inhibited syncytium formation between HIV-1IIIB/H9 cells and C8166 cells with an IC50 value of 0.33 µg/L. The IC50 of sifuvirtide was approximately 50-fold lower than that of enfuvirtide (16.8 µg/L). The results suggested that sifuvirtide was more effective than enfuvirtide in blocking HIV-1 entry into cells.
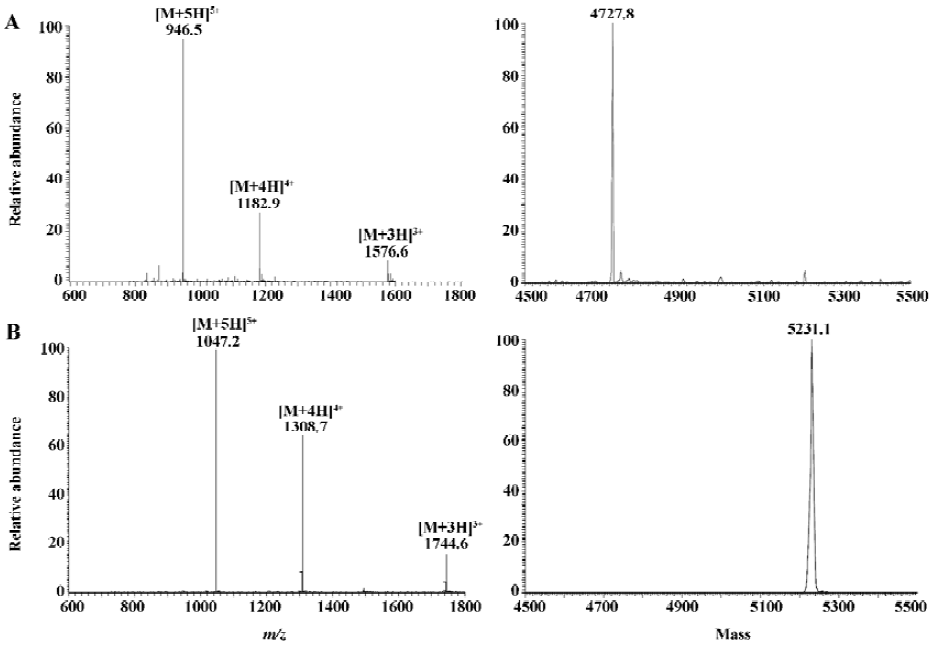
Mass spectrometry detection of sifuvirtide and 127I4-sifuvirtide The mass spectrum obtained by infusing 10 mg/L solution of sifuvirtide and 127I4-sifuvirtide in water:ACN (50:50, v/v) at a flow rate of 5 µL/min is shown in Figure 1A. A series of multiply charged ions, [M+5H]5+ at m/z 946.5, [M+4H]4+ at m/z 1182.9 and [M+3H]3+ at m/z 1576.6 for sifuvirtide, and [M+5H]5+ at m/z 1047.2, [M+4H]4+ at m/z 1308.7 and [M+3H]3+ at m/z 1744.6 for 127I4-sifuvirtide, were observed in positive ion mode. Deconvoluted mass obtained automatically utilizing the Bioworks software (Ver 3.1 SR1, Thermo-Electron, San Jose, CA, USA) showed a difference of 503.3 u (theoretical value was 503.6 u) between sifuvirtide and 127I4-sifuvirtide, which demonstrated that a complete iodination had been performed and reached the goal product. The most abundant ions of sifuvirtide and 127I4-sifuvirtide, [M+5H]5+ at m/z 946.5 and m/z 1047.2, respectively, were selected as parent ions for a collision-induced dissociation experiment. The most intensive product ions for sifuvirtide and IS were observed at m/z=871.87 (b335+) and m/z=972.6 (b335+), respectively, both with optimized normalized collision energy of 28%[10].
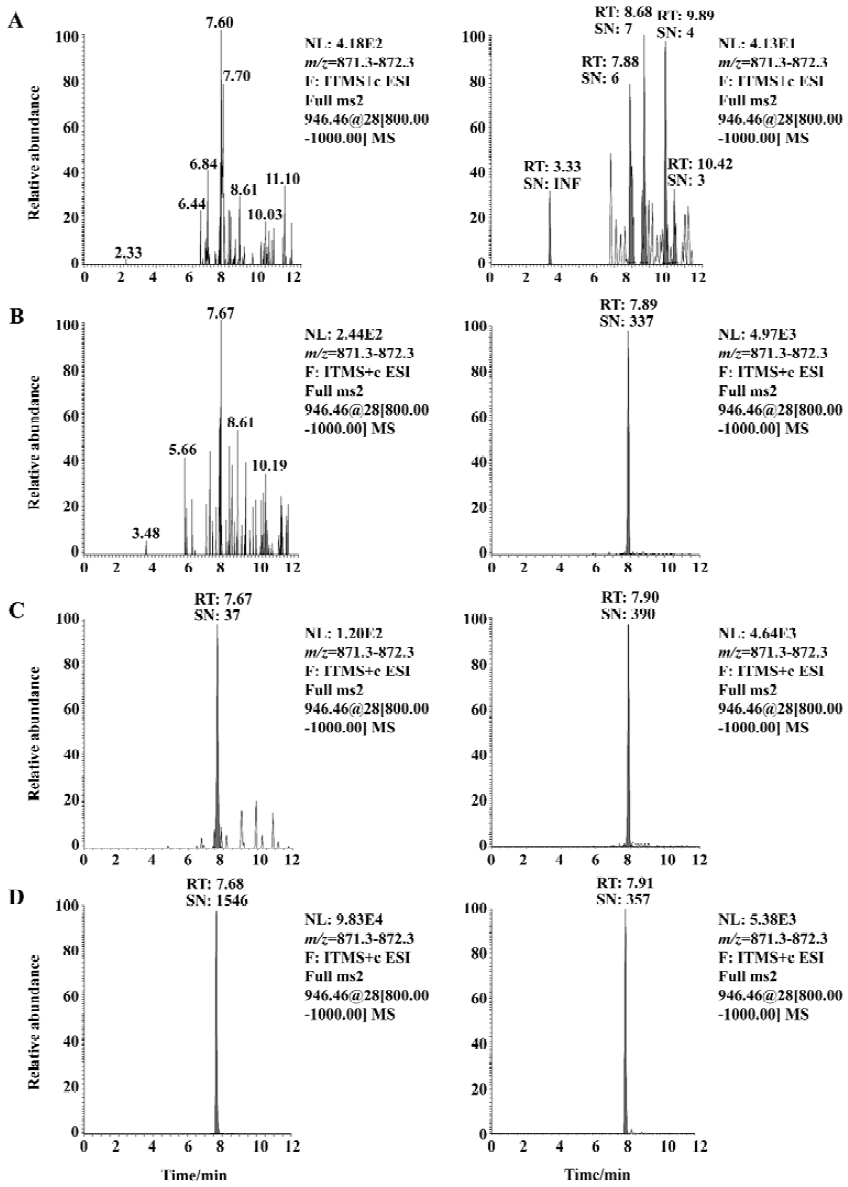
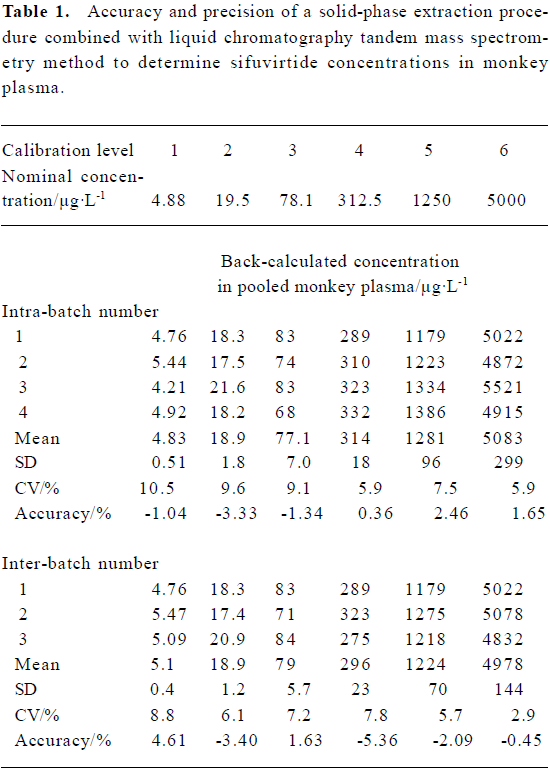
Method validation Under the established SPE-LC/MS/MS conditions, the typical retention times for sifuvirtide and IS were 7.68 min and 7.91 min, respectively, without interference from endogenous plasma components. The limit of detection was 1.22 µg/L, which represented 122 pg on-column injected analyte (approximately 26 fmol in drug-free monkey plasma, absolute recovery was seen as 100%), with a signal to noise (S/N) ratio above 10. The typical chromatograms of sifuvirtide in monkey plasma are illustrated in Figure 2. Calibration curves exhibited excellent linearity over a range of 4.88 μg/L to 5000 µg/L, and the typical regression equation for sifuvirtide was y=0.01736?0.003759x, with a r2 of 0.9929. The intra-assay and inter-assay precision and accuracy values of the method were assessed using spiked plasma samples at whole calibration concentration levels for sifuvirtide, shown in Table 1. The mean accuracy of back-calculated concentrations of the standards compared with theoretical ones ranged from -5.36% to 4.61%. The inter-batch CV ranged from 2.9% to 8.8%, and the intra-batch CV did not exceed 10.5%. The stability of sifuvirtide in solution and in plasma samples was also evaluated[10]. The areas ratios of SRM chromatography spectra of sifuvirtide versus IS were investigated in stock solution kept in 4°C for approximately 15 d and in plasma samples left at 15°C overnight (12 h-14 h). No real change in the area ratio of sifuvirtide to IS was observed.
Full table
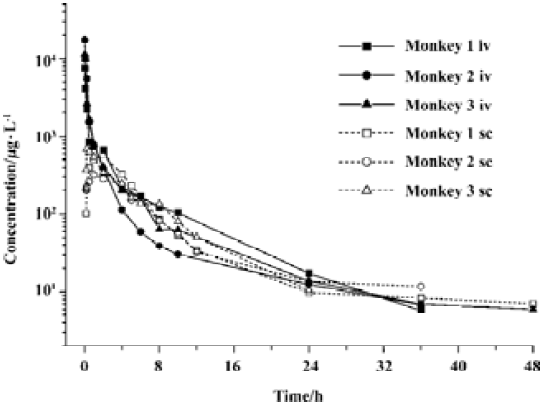
Phamacokinetics of sifuvirtide in rhesus monkeys The individual concentration-time profiles of sifuvirtide after iv or sc administration are shown in Figure 3. Plasma concentration versus time data was analyzed and the PK parameters are shown in Table 2. After administration of sifuvirtide at doses of 1.2 mg/kg, the observed peak concentration was 10 626±2886 µg/L for iv at 1 min, and 528±191 µg/L for sc at 0.25 h-2 h, respectively. The terminal elimination plasma half-lives (T1/2) after iv or sc were 6.3±0.9 h and 5.5±1.0 h, respectively. Following sc administration, sifuvirtide was absorbed and distributed rapidly, and meanwhile was eliminated quickly in plasma. The bioavailability after sc injection was 49%±13%. In the present study, a remarkable inter-individual variability of the PK parameters, such as AUC, Cmax, Tmax, and Fabs, of sifuvirtide was observed in both iv and sc administration groups.
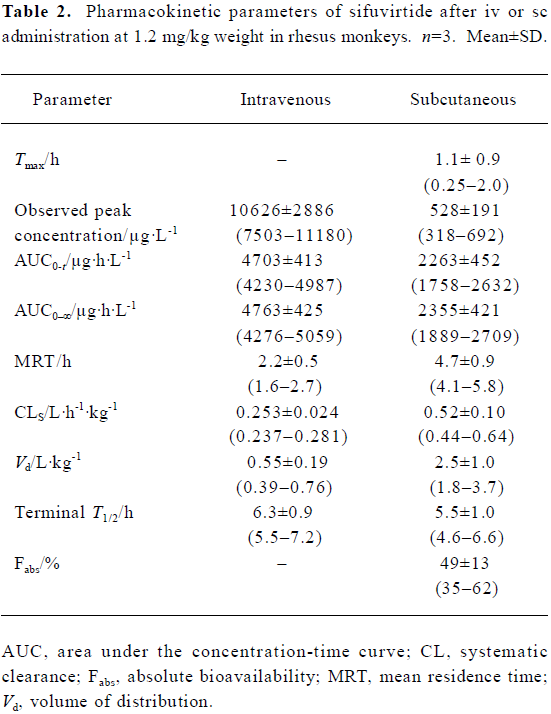
Full table
Discussion
In the present study, the advanced on-line SPE-LC/MS/MS system was validated as being reliable, reproducible, and highly sensitive for the quantitative determination of sifuvirtide in monkey plasma. The programmable column-switching technique was the core of this fully automatic system. Based on the design of on-line splitting injection mode (OSIM), this novel on-line SPE system showed a powerful performance and capability for sample preparation of more than 300 raw plasma samples. The addition of pH adjusting solvent is very important for increasing the extraction efficiency of sifuvirtide. Coupled with LTQ-MS, a novel quadrupole ion trap mass spectrometer, a satisfactory sensitive and good calibration curves yielding a wide linearity range over 3 orders of magnitude with correlation coefficients higher than 0.9923 were obtained.
Due to the lack of effective vaccines for the prevention of HIV infection, development of potent and affordable anti-HIV drugs is an international priority. As the first fusion inhibitor, enfuvirtide has been considered to have the disadvantages of short elimination half-life (2.5 h-3.5 h in the monkey via sc route[14]), which is administered twice at a high cost. A lower IC50 value of sifuvirtide for cell-to-cell transmission inhibition of HIV-1 in vitro was determined at around 0.33 µg/L, which is lower than that of enfuvirtide (16.8 µg/L). Therefore, a relatively lower dosage of sifuvirtide is recommended for future clinical treatments. In addition, the PK parameters of sifuvirtide after sc or iv dosing at 1.2 mg/kg weight in rhesus monkeys were below those of enfuvirtide (0.8 mg/kg in cynomolgus monkey[14]). Although the average observed concentration of sifuvirtide at 10 min after dosing was lower than that of enfuvirtide, the plasma concentration profiles of sifuvirtide after iv or sc were sustained at a relatively higher ratio to the in vitro IC50, even at 36 h after dosing. The terminal half-life of sifuvirtide was estimated as 5.5 h-7.2 h after iv and 4.6 h-6.6 h after sc administration, which is longer than that of enfuvirtide. A lower average AUC0-∞and higher mean Vd and CL (normalized by animal weight) was found for sifuvirtide compared with enfuvirtide, which indicated that sifuvirtide was widely distributed to tissues or organs via circulation of blood and showed the benefit of antiviral action in vivo. These results were in accordance with those derived from tissue distribution experiments by radioactive determination (data not shown). The comparative MRT and Fabs (%) of two peptides were estimated in monkeys by the non-compartmental approach. All of these results derived from inhibitory activity in vitro and PK characteristics provide an important reference for future clinical studies.
Acknowledgements
We thank Dr Zhi-yun MENG and Mr Jun LI for their helpful discussions and excellent technical support.
References
- Locatelli GA, Cancio R, Spadari S, Maga G. HIV-1 reverse transcriptase inhibitors: current issues and future perspectives. Curr Drug Metab 2004;5:283-90.
- Imamichi T. Action of anti-HIV drugs and resistance: reverse transcriptase inhibitors and protease inhibitors. Curr Pharm Des 2004;10:4039-53.
- Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, et al. AIDS across Europe, 1994?98: the EuroSIDA study. Lancet 2000;356:291-6.
- Li Y, McDonald AM, Dore GJ, Kaldor JM. Improving survival following AIDS in Australia, 1991?1996. National HIV Surveillance Committee. AIDS 2000;14:2349-54.
- Stolk LM, Luers JF. Increasing number of anti-HIV drugs but no definite cure. Review of anti-HIV drugs. Pharm World Sci 2004;26:133-6.
- Ruprecht RM, Ferrantelli F, Kitabwalla M, Xu W, McClure HM. Antibody protection: passive immunization of neonates against oral AIDS virus challenge. Vaccine 2003;21:3370-3.
- Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA 1994;91:9770-4.
- Williams IG. Enfuvirtide (Fuzeon): the first fusion inhibitor. Int J Clin Pract 2003;57:890-7.
- Steinbrook R. HIV infection朼 new drug and new costs. N Engl J Med 2003;348:2171-2.
- Dai SJ, Song HF, Dou GF, Qian XH, Zhang YJ, Cai Y, et al. Quantification of sifuvirtide in monkey plasma by an on-line solid-phase extraction procedure combined with liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 2005;19:1273-82.
- Tejedor F, Ballesta JP. Iodination of biological samples without loss of functional activity. Anal Biochem 1982;127:143-9.
- Wang Q, Wang YT, Pu SP, Zheng YT. Zinc coupling potentiate anti-HIV-1 activity of baicalin. Biochem Biophys Res Commun 2004;324:605-10.
- Dunne A. Statistical moments in pharmacokinetics: models and assumptions. J Pharm Pharmacol 1993;45:871-5.
- US Food and Drug Administration [homepage on the internet]. Rockville MD: Food and Drug Administration; 2003 [date created 2003 Jul 23; cited 2005 Feb, 19]. Available from: http://www.fda.gov/cder/foi/nda/2003/021481_fuzeon_review.htm
