Deguelin regulates nuclear pore complex proteins Nup98 and Nup88 in U937 cells in vitro1
Introduction
The nuclear pore complex (NPC) comprises a central eightfold symmetrical ring and spoke assembly, cytoplasmic fibers, and a filamentous nuclear basket[1]. Molecular trafficking between the nucleus and the cytoplasm of interphase cells occurs via the NPC, which are large molecular assemblies that are embedded in the double-membraned nuclear envelope (NE)[2]. The NPC provide peripheral channels of approximately 9 nm in diameter, which allow the diffusion of ions and small molecules, and mediate the selective transport of nuclear proteins, RNA, and ribonucleoprotein (RNP) particles by energy-dependent mechanisms. Several interactions between individual FG (Phe-Gly) repeat-containing nucleoporins and transport factors have been reported, leading to the idea that such interactions may play a pivotal role in the docking, translocation, and/or termination steps of the transport process[3]. Recent research has shown that Nup98 can dynamically associate with the nuclear pore and shuttle between the NPC and intranuclear bodies, and additionally between the nucleus and the cytoplasm in a transcription-dependent manner. The most common oncogenic fusions involve a segment of the gene encoding the FG-repeat domain of Nup98, which, in turn, becomes linked to genes of the homeobox family of transcription factors[4]. Nucleoporins are involved in several types of acute myeloid leukemia and a few other hematological malignancies, as well as rare cases of other tumors. Overexpression of nucleoporin 88 (Nup88) is associated with malignant tumors, whereas in most other cases the role of the Nup proteins in tumorigenesis stems from chromosomal rearrangements that results in oncogenic fusion proteins[5]. Nup98 and Nup88 play important roles in nucleocytoplasmic shuttling activity in carcinoma cells.
Several natural compounds, in particular plant products and dietary constituents, have been found to have chemo-preventive activities both in vitro and in vivo[6]. Deguelin has been isolated from several plant species, including Mundulea sericea (Leguminosae). Recent experiments have verified that deguelin can lead the cell cycle to block and induce apoptosis; however, the mechanism by which it acts is not yet completely clear[7–9]. In our previous studies, we found that deguelin was able to inhibit the proliferation of Burkitt’s lymphoma cell line Daudi cells by regulating the cell cycle such that cells were arrested at the G0/G1 phase, and apoptosis was induced. Moreover, deguelin has low toxicity in human peripheral blood monocular cells (PBMC), but selectively induces the apoptosis of Daudi cells. Deguelin has antitumor effects because it downregulates the expression of cyclin D1 and the pRb protein[10]. In the present study, we chose human myeloid precursor cell line U937 cells as the target. This study was designed to explore the mechanism by which deguelin regulates Nup98 and Nup88 in U937 cells. We focused on changes in the expression of Nup98 and Nup88, and analyzed the underlying mechanism by which molecular trafficking between the nucleus and the cytoplasm is carried out.
Materials and methods
Drugs and reagents Deguelin was purchased from the Sigma (St Louis, MO, USA) and was initially dissolved in dimethylsulfoxide (Me2SO), and stored at -20 °C, and was then thawed before use. 3-(4,5-dimethyl-2 thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was purchased from Janssen Chimica Company (New Brunswick, NJ, USA). RPMI-1640 medium, propidium iodide (PI), Hoechst 33258, and Me2SO were purchased from Sigma. Fetal calf serum (FCS), anti-Nup88 and anti-Nup98 antibodies were purchased from Santa Cruz (California, USA). Fluorescein isothiocyanate (FITC)-labeled secondary antibodies were purchased from Zhongshan Company (Beijing, China). Chemiluminescence (ECL) reagent kits were purchased from Pierce Biotechnology (Rockford, IL, USA). The U937 cell line was obtained from the China Center for Typical Culture Collection (Wuhan, China). All cell groups were grown in an RPMI-1640 culture medium containing 10% FCS and 2 mmol/L L-glutamine at 37 ºC in a 5% CO2 incubator.
MTT assay The antiproliferative effect of deguelin against different group cells was determined by using the MTT dye uptake method as described previously[11]. Briefly, the final concentrations of deguelin were 0, 5, 10, 20, 40, 80, and 160 nmol/L. Each concentration of deguelin was added to 6 wells, respectively. The plates were in the presence or absence of the indicated test samples for 0 h, 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h. Thereafter, 20 µL MTT solution [5 mg/mL in phosphate-buffered saline (PBS)]was added to each well. After incubation for 4 h at 37 °C, the supernatant was removed and 150 µL Me2SO was added. When the blue crystals were dissolved, the optical density (OD) was detected in a microplate reader at a wavelength of 570 nm using a 96-well multiscanner autoreader (Biotech Instruments µQuant, NY, USA). The following formula was used: cell proliferation inhibited (%)=[1–(OD of the experimental samples/OD of the control)]×100% (n=6. Mean±SD).
DNA content and cell cycle analysis Untreated and treated cells were collected, after being cultured in the presence or absence of deguelin for the indicated time, rinsed with PBS, and suspended in 75% ethanol at -20 ºC overnight. Fixed cells were centrifuged at 1200×g and washed twice with PBS. For detecting DNA content, cells were incubated in the dark with 50 mg/L PI and 0.1% RNase A in 400 µL PBS at room temperature for 30 min. Stained cells were analyzed using FACSort (Becton Dickinson, New Jersey, USA). The percentage of cells was determined using the CellQuest software program (Becton Dickinson, New Jersey, USA). Cells were grouped as follows: the control group; and those treated with deguelin at concentrations of 5, 10, 20, 40, and 80 nmol/L for 24 h, respectively (n=3).
Immunofluorescence with confocal microscopy For the immunofluorescence experiments, cells were fixed in 4% paraformaldehyde for 10 min and permeabilized with 0.2% Triton X-100 on ice for 10 min. Samples were blocked with 3% bovine serum albumin plus 0.02% Triton X-100 in PBS for 30 min, incubated with anti-Nup88 (1:200) and anti-Nup98 (1:100) antibodies overnight at 4 ºC, and washed 4 times with 1.5% bovine serum albumin plus 0.02% Triton X-100 in PBS. FITC-labeled secondary antibodies diluted in PBS were applied for 30 min, and cells were washed 3 times every 15 min. Hoechst 33258 (1 µg/mL) and PI (50 mg/L) were included in the penultimate wash step to visualize the DNA. Coverslips were mounted with 3-amino propyltriethoxy silane (APES). Images were captured using a FV500 confocal microscope (Olympus, Tokyo, Japan).
Nup98 and Nup88 protein analysis using flow cytometry Flow cytometry was performed to determine the expression of Nup98 and Nup88 in cells by using primary antibodies to the peptide-binding domain. A total of 1×106 cells were collected, washed with PBS, and anti-Nup88 antibody (1:50) and anti-Nup98 antibody (1:50) were added, then the mixture was kept at 4 ºC overnight. Mouse IgG1 (1:50) antibody was the isotype control group. FITC-labeled secondary antibody diluted in PBS (1:100) was applied for 30 min at room temperature. Stained cells were analyzed by using FACSort. A total of 10 000 cells were analyzed from each cell group. The percentage of cells was determined using the CellQuest software program. Cells were grouped as follows: the negative group; the blank group; those treated with 10 nmol/L deguelin for 24 h; those treated with 20 nmol/L deguelin for 24 h.
Immunoelectron microscopy U937 cells on coverslips were fixed in 4% paraformaldehyde and 0.2% glutaraldehyde and washed with 0.1 mol/L phosphate buffer (PB; pH 7.4), followed with PB containing 0.1% sodium borohydride to inactivate residual aldehyde groups. The cells were permeabilized with PB containing 0.05% Triton X-100 for 20 min at room temperature and washed with PB. The blocking solution was PBS (pH 7.4) containing 4% normal goat serum (NGS). After blocking, cells were incubated with affinity-purified goat anti-Nup98 antibody (1:100) and affinity-purified mouse anti-Nup88 antibody (1:100) in PBS containing 1% NGS at 4 ºC overnight. After 6 washes with PBS, cells were incubated with a biotinylated respondent secondary antibody (1:200) in PBS and 1% NGS. Immunoreactivity was visualized by incubation with 0.05% diamino-benzidine (DAB; Sigma) and 0.003% hydrogen peroxide in 0.05 mol/L Tris (pH 7) for 2 min. Cells were washed, postfixed with 2.5% glutaraldehyde in PB, washed again, fixed with 0.5% osmium tetroxide for 15 min, dehydrated, and embedded in Leica ultracut UCT (Wetzlar, Germany) for sectioning. Sections were observed on an electron microscope (Tecnai F12; FEI, Eindhoven, the Netherlands).
Western blot analysis Lysates were prepared from 1×107 cells by dissolving cell pellets in 100 µL of lysis buffer [20 mmol/L Na2PO4 (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 1% aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, 10 g/L leupeptin, 100 mmol/L NaF, and 2 mmol/L Na3VO4]. Lysates were centrifuged at 18 000×g for 15 min and the supernatant was collected. Protein content was determined using a Bio-Rad protein assay (Bio-Rad laboratories, Hercules, CA, USA). Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer [10 mmol/L Tris-HCl (pH 6.8) 2% SDS, 10% glycerol, 0.2 mol/L 1,4-Dithiothreitol, (DTT)] was added to the lysates. Lysates were heated to 100 ºC for 5 min, and 100 µg of protein was loaded into each well of a 10% SDS-PAGE gel. Resolved proteins were electrophoretically transferred to nitrocellulose and blocked with 5% non-fat milk. After incubation with the Nup88 antibody (at a 1:2500 dilution) and the Nup98 antibody (dilution 1:1000) at 4 °C overnight, the blots were washed 3 times with TBS/Tween [TBST; 25 mmol/L Tris-HCl (pH 8.0), 125 mmol/L NaCl, 0.1% Tween 20], and exposed to horseradish peroxidase (HRP)-conjugated corresponding secondary antibodies for 1 h, and finally detected by using ECL. Quantification of the bands was carried out using the Quantity One densitometric analysis software (Bio-Rad).
Statistical analysis All data are expressed as mean±SD, and analyzed using SPSS 10.0 for Windows 98. Linear t-test were used for statistical analyses, and P<0.05 was considered to be statistically significant.
Results
Effects of deguelin on proliferation of U937 cells U937 cells treated with different concentrations of deguelin (0, 5, 10, 20, 40, 80, or 160 nmol/L) for 0 h, 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h, respectively, resulted in the inhibition of cell proliferation in a dose- and time-dependent manner. The OD value of the deguelin-treated group was significantly lower than that of the untreated group (Figure 1). The IC50 value for 24 h for the U937 cells was 21.61 nmol/L, whereas the IC50 value for 36 h was 17.07 nmol/L.
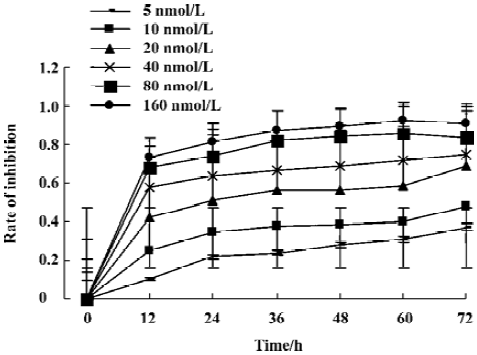
Effects of deguelin on the cell cycle of human leukemia U937 cells Figure 2 illustrates the changes in DNA content distribution in cells treated with 0, 5, 10, 20, 40, or 80 nmol/L deguelin for 24 h. As the treatment dose increased, the percentage of cells in S phase and G2/M phase increased, whereas the number in the G1/G0 phase decreased accordingly. After treatment with 0, 5, 10, 20, 40, or 80 nmol/L deguelin for 24 h, the proportion of cells in the G1/G0 phase were 73.01%, 71.15%, 68.42%, 53.83%, 43.99%, and 22.82%, respectively; the proportion decreased in a dose-dependent manner. The proportion of S phase cells were 17.18%, 16.30%, 18.09%, 27.56%, 31.21%, and 46.85%, respectively; the proportion increased in a dose-dependent manner. The proportion in the G2/M phase was 9.75%, 12.31%, 13.99%, 18.99%, 24.83%, and 27.79%, respectively. These results show that deguelin arrested the U937 cells at the S phase and the G2/M phase, and decreased the number of cells in the G1/G0 phase in vitro.
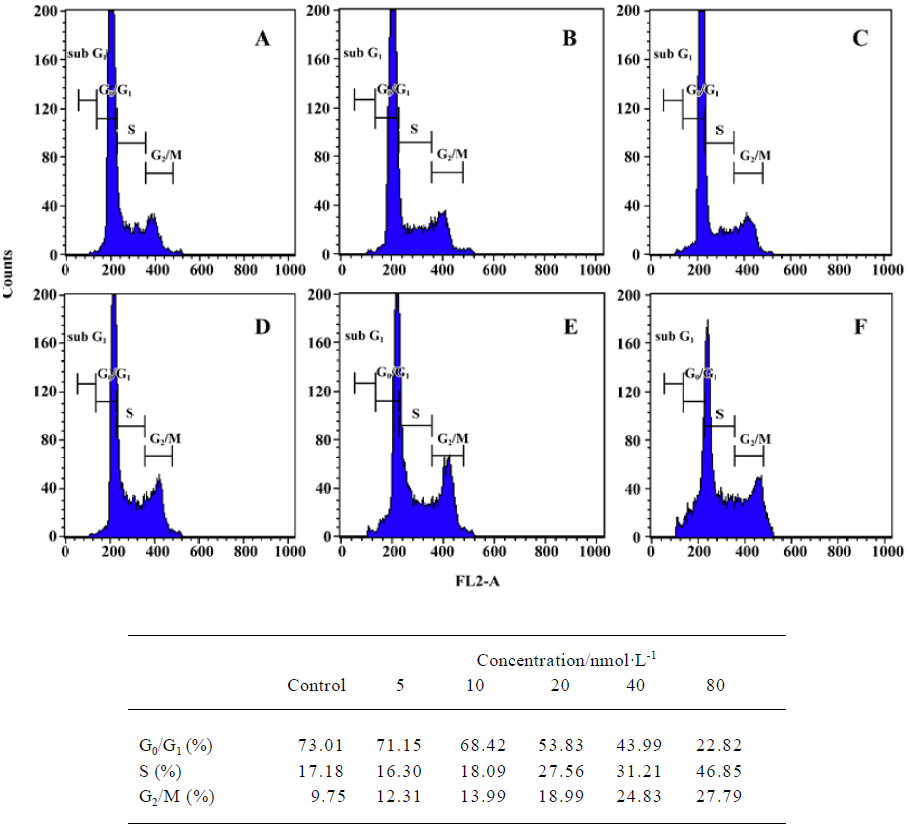
Effects of deguelin on Nup98 and Nup88 in U937 cells In this study, FITC-labeled secondary antibodies marked Nup98 and Nup88 with green fluorescence, and Hoechst 33258 and PI labeled the DNA of U937 cells with blue and red fluorescence, respectively. In Figure 3, part Ad is merged parts Ab and Ac, and part Bd is merged parts Bb and Bc using confocal microscopy. We found that Nup98 had low fluorescence intensity, with an OD value of 50.23 in U937 cells, and the fluorescence was located both within the nucleoplasm and nucleus in intensely fluorescent dots. After treatment with 20 nmol/L deguelin for 24 h, Nup98 had greater fluorescence intensity, with an OD value of 252.28, and its distribution was mainly within the nucleus. In U937 cells, Nup88 had high fluorescence intensity, with an OD value of 215.16. After treatment with 20 nmol/L deguelin for 24 h, Nup88 had lower fluorescence intensity, with an OD value of 63.24. Compared with the control group, expression of Nup98 and Nup88 after treatment with 20 nmol/L deguelin for 24 h was significantly different (P<0.05). When these data are considered together, we conclude that Nup98 and Nup88 are regulated by deguelin, but the mechanism by which this occurs is not completely clear.
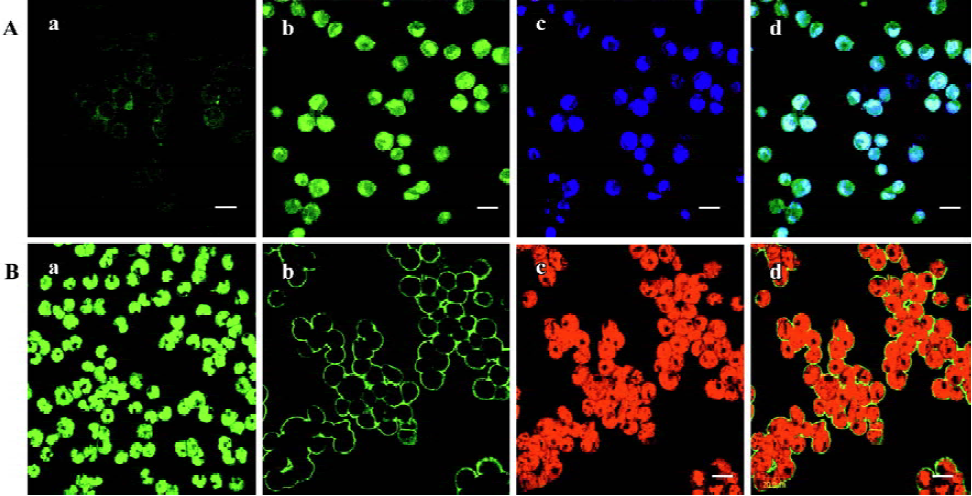
Nup98 and Nup88 protein analysis in U937 cells We used flow cytometry to measure the expression of Nup98 and Nup88 in untreated U937 cells, and in U937 cells treated with deguelin. In untreated cells, the average fluorescence intensity of Nup98 in the blank controls was 23.55. In cells treated with 10 nmol/L and 20 nmol/L deguelin for 24 h, the average fluorescence intensity of Nup98 was 189.58 and 249.32, respectively. The average fluorescence intensity of Nup88 in the blank controls was 53.92. When cells were treated with 10 nmol/L and 20 nmol/L deguelin for 24 h, the average fluorescence intensity of Nup88 was 11.12 and 10.03, respectively (Figure 4; n=3).
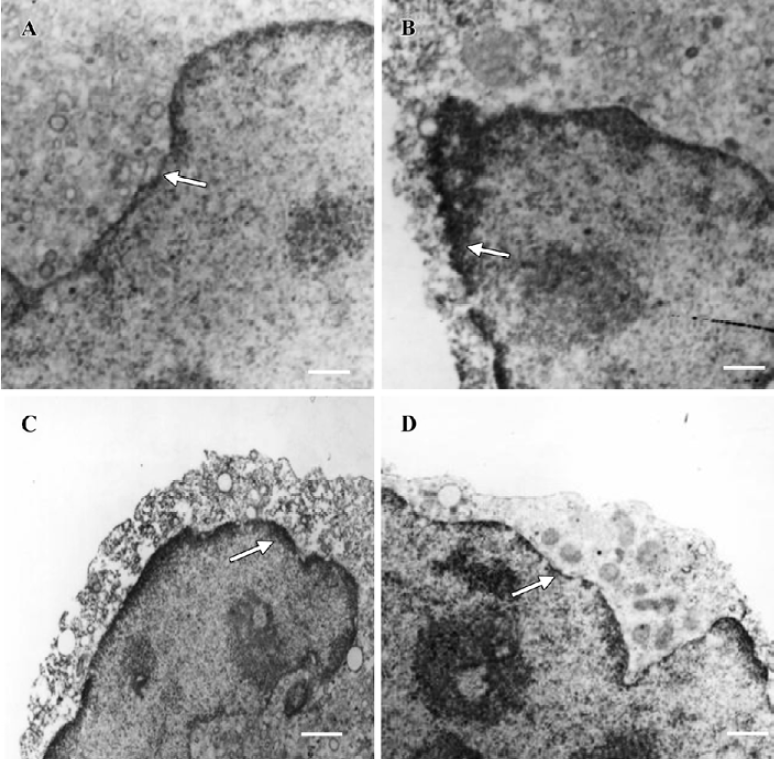
Location of Nup98 and Nup88 in U937 cells To visualize the subcellular location of Nup98 and Nup88, untreated and treated U937 cells were detected by using immunoelectron microscopy. In Figure 5, the nuclear pore proteins are indicated by arrowheads and are mainly located within the nuclear membrane. The density of the nuclear pore protein is based on DAB staining. In Figure 5, the electronic density of Nup98 in untreated cells is lower than that in deguelin-treated U937 cells. The electronic density of Nup88 in untreated cells was higher than that in deguelin-treated U937 cells.
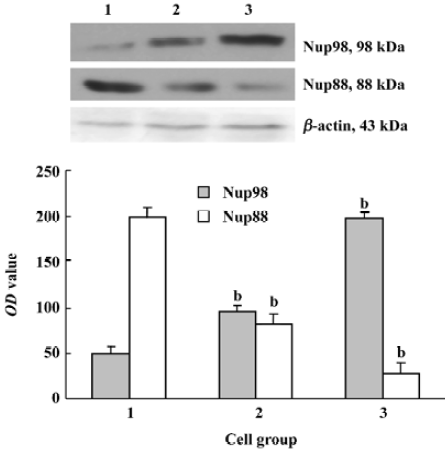
Expression of Nup98 and Nup88 in U937 cells Our results reveal that deguelin can induce antiproliferation and apoptosis in U937 cells. However, it is unclear how U937 induces these effects. U937 cells treated with 10 nmol/L and 20 nmol/L of deguelin for 24 h were lysed and resolved using 10% SDS-PAGE, and then Western blot analysis was carried out using anti-Nup98 and anti-Nup88. Figure 6 shows the marked change in Nup98 and Nup88 expression following deguelin treatment. Deguelin is related to upregulating the expression of Nup98 and downregulating the expression of the Nup88 protein. This indicates that Nup98 and Nup88 are related to the deguelin-mediated nucleocytoplasm shuttling activity, which is related to cell proliferation and apoptosis.
Discussion
Deguelin, a natural plant extract, is commonly used as an insecticide in Africa, South America and China. Deguelin belongs to a class of compounds called rotenoids, which have chemopreventive activity[6]. In fact, deguelin has already been shown to prevent skin and breast tumors in experimental models[7]. It has chemopreventive activity, and acts by inhibiting NADH:ubiquinone oxidoreductase activity, and by regulating the cell cycle and inducing apoptosis[7]. Chun et al found that deguelin inhibited the growth of and promoted apoptosis of premalignant and malignant cells[8]. In contrast, the compound had little effect on normal HBE cells. The drug’s antineoplastic effects and specificity appeared to be due to its ability to inhibit phosphatidylinositol 3-kinase (PI3K)/Akt-mediated signaling pathways[9,10]. Bortul et al found that deguelin enhanced the sensitivity of U937 leukemia cells and acute myeloid leukaemia (AML) blasts to chemotherapeutic drugs with an activated PI3K/Akt network[10]. In the present experiment we found that deguelin suppressed the proliferation of U937 cells, and that deguelin may have potential as an anti-tumor medicine. After treatment with different doses of deguelin, U937 cells accumulated in the S and G2/M phases, whereas the number of cells in the G0/G1 phase decreased in a dose-dependent manner. We also found that deguelin upregulated the expression of Nup98 and downregulated the expression of Nup88 in U937 cells. This suggests that changes in the ratio of Nup98 and Nup88 might contribute to the apoptosis-promoting activity of deguelin in these cells. The changes effected in nucleoporin in U937 cells by deguelin offer new possibilities for exploring the underlying anti-tumor mechanism of deguelin.
The various events of nuclear division, cytoplasmic division, cell growth, and cell maturation are repeated in each generation of cells[12]. The periods of time and the sequence of events from one cell division to the next are collectively referred to as the cell cycle. Severe defects in chromosomes block progression through the cell cycle, and can lead to cell suicide or apoptosis[13]. Deguelin plays an important pharmacological role by acting on different stages of the cell cycle in tumor cells. In this study, U937 cells were arrested mainly in the S and G2/M phases by deguelin, whereas the proportion of cells in the G0/G1 phase gradually declined. After treatment with different doses of deguelin for 24 h, cells in the G0/G1 phase were earliest influenced, and the proportion of cells in this phase decreased gradually in a dose-dependent manner. At the same time, the proportion of cells in the S phase increased gradually, with treatment with 80 nmol/L deguelin resulting in the highest value. The proportion of cells in the G2/M phase increased in a dose-dependent manner. These data show that deguelin usually regulates the G1/S and G2/M checkpoints in U937 cells. Cell cycle checkpoint controls at the G1 to S transition and the G2 to M transition prevent the cell cycle from progressing when DNA is damaged[14]. That deguelin regulates the cell cycle checkpoints has been verified in other tumor cells. Chun et al found that after treatment with deguelin, the proportion of premalignant cells in the G0/G1 phase increased and the proportion of malignant HBE cells in the G2/M phase increased from 9.6% to 40.2%[8]. Bortul et al found that deguelin (10 nmol/L) induced S phase arrest by interrfering with the progression to G2/M[10]. In our previous studies [11], we found that deguelin was able to inhibit the proliferation of Daudi cells by regulating the cell cycle that arrests cells at the G0/G1 phase, and had no effect on the G2/M phase. The mechanism by which deguelin causes these different cell cycle changes is not yet completely clear, but it is mainly related to the sensitivity of tumor cells to deguelin.
Transport between the nucleus and the cytoplasm occurs through NPC embedded in the nuclear envelope. Nucleoporins are involved in several types of acute myeloid leukemia and a few other hematological malignancies, as well as rare cases of other tumors[15]. An expanding subgroup of chromosomal translocation-generated oncoproteins in human acute myeloid leukemias (AML) involve the FG repeat-containing NPC proteins Nup98 and CAN/Nup214[16]. The Nup98 gene is found at the breakpoints of two distinct chromosomal rearrangements: t (7;11)(p15;p15) and inv(11)(p15;q22), which link Nup98 to the class I homeotic transcription factor HOXA9 and the putative RNA helicase DDX10, respectively. The most common oncogenic fusions involve a segment of the gene encoding the FG-repeat domain of Nup98, which, in turn, becomes linked to genes of the homeobox family of transcription factors. The Nup98-derived FG-repeat segments of the resulting oncogene interact with the transcriptional coactivators CBP (CREB binding protein) and P300, thereby leading to increased gene transcription[17]. FG-repeat segments of two other nucleoporins, Nup153 and CAN/Nup214, can substitute for the Nup98 segment in the oncogenic fusion protein. When Nup98 is disrupted, it selectively impairs discrete protein import pathways, which supports the idea that transport of distinct import complexes through the NCP is mediated by specific subsets of nucleoporins. Because Nup98 plays a role in RNA export, its mobility suggests that Nup98 might associate with RNA close to its transcription site and then further accompany the processed RNA through the NPC into the cytoplasm[18]. Using confocal microscopy, we found that Nup98 was present on both sides of the NPC in U937 cells, and was localized inside the nucleus. When cells were treated with 10 nmol/L and 20 nmol/L deguelin for 24 h, the average fluorescence intensity of Nup98 was 189.58 and 249.32. Deguelin can regulate the expression of Nup98, and this shows that deguelin participates in Nup98 leading nucleocytoplasmic traffic.
Nup88 is a NCP protein; the Nup88 gene is localized at 17p13 and the Nup88 protein is involved in nuclear–cytoplasmic transport and cell growth[19]. Overexpression of Nup88 has been found in human tumors of the stomach, colon, liver, pancreas, breast, lung, ovary, uterus, prostate and kidney[20]. The Nup88 protein is also overexpressed in malignant tumor tissue relative to normal surrounding tissue. Recent studies have found that the Nup88 protein is enhanced in most metastatic melanomas relative to their corresponding primary tumors[21]. Both RNA transcription and protein expression levels are higher in malignant tumor cell lines compared with non-transformed cells. The Nup88 protein is strongly expressed in the invasive margins of both primary and metastatic breast, endometrial and colorectal carcinomas[22]. Overexpression of Nup88 in malignant tumors is probably due to the enhanced nucleocytoplasmic transport required to meet the increased demand for proteins in the tumor cells. Nup88 is considered to be a tumor growth factor, and is a positive regulator in the cell cycle. For this reason, we devised the present study on Nup88 expression in U937 cells and studied the regulative effects of deguelin on Nup88. Our data support the idea that Nup88 might be involved in the tumor progression of U937 cells, and these findings show that deguelin participates in Nup88 leading nucleocytoplasmic traffic.
In summary, our results show that deguelin can inhibit U937 cell proliferation in a dose- and time-dependent manner, with an IC50 value for 24 h of 21.61 nmol/L and an IC50 value for 36 h of 17.07 nmol/L. Deguelin usually regulates the G1/S and G2/M checkpoints. In addition, deguelin causes upregulation of the expression of Nup98, and downregulation of the Nup88 protein in U937 cells. The reasons for this effect, and the mechanisms for this regulation of Nup98 and Nup88, and whether these mechanisms are linked to inhibited cell proliferation, are unknown, but our findings suggest that Nup98 and Nup88 may be involved in nucleocytoplasmic shuttling in carcinoma cells.
Acknowledgements
We thank the Tumour Biology Laboratory, Center of Gynaecology, Tongji Hospital, Huazhong University of Science and Technology, China, for offering relevant experimental facilities and technical support. We wish to particularly thank Prof Yun-ping LU for guidance and help with the experiment.
References
- Frosst P, Guan T, Subauste C, Hahn K, Gerace L. Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export. J Cell Biol 2002;156:617-30.
- Enninga J, Levy DE, Blobel G, Fontoura BM. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 2002;295:1523-5.
- Griffis ER, Xu SL, Powers MA. Nup98 localizes to both nuclear and cytoplasmic sides of the nuclear pore and binds to two distinct nucleoporin subcomplexes. Mol Biol Cell 2003;14:600-10.
- Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol Biol Cell 2002;13:1282-97.
- Nerea M, Angel A, Moragues MD, Jose P, Jose S. The nuclear pore complex protein Nup88 is overexpressed in tumor cells. Cancer Res 1999;59:5408-11.
- Udeani GO, Gerhauser C, Thomas CF, Moon RC, Kosmeder JW, Kinghorn AD, et al. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer Res 1997;57:3424-8.
- Lee HY, Suh YA, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells. Clin Cancer Res 2004;10:1074-9.
- Chun KH, Kosmeder JW 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK, et al. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst 2003;95:291-302.
- Murillo G, Salti GI, Kosmeder JW, Pezzuto JM, Mehta RG. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur J Cancer 2002;38:2446-54.
- Bortul R, Tazzari PL, Billi AM, Tabellini G. Deguelin, a PI3K/AKT inhibitor, enhances chemosensitivity of leukaemia cells with an active PI3K/AKT pathway. Br J Haematol 2005;129:677-86.
- Liu HL, Chen Y, Cui GH, Wu QL, He J. Experimental study on regulating expressions of cyclin D1, pRb, and anticancer effects of deguelin on human Burkitt’s lymphoma Daudi cells in vitro. Acta Pharmacol Sin 2005;26:873-80.
- Liu HL, Chen Y, Cui GH, Zhou JF. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation effect. Acta Pharmacol Sin 2005;26:603-9.
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411:342-8.
- Sun SY, Hail N Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst 2004;96:662-72.
- Pietenpol JA, Stewart ZA. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology 2002;181:475-81.
- Hodel AE, Hodel MR, Griffis ER, Hennig KA, Ratner GA, Xu S, et al. The three-dimensional structure of the autoproteolytic, nuclear pore-targeting domain of the human nucleoporin Nup98. Mol Cell 2002;10:347-58.
- Gould VE, Martinez N, Orucevic A, Schneider J, Alonso A. A novel, nuclear pore-associated, widely distributed molecule overexpressed in oncogenesis and development. Am J Pathol 2000;157:1605-13.
- Kobzev YN, Martinez-Climent J, Lee S, Chen J, Rowley JD. Analysis of translocations that involve the NUP98 gene in patients with 11p15 chromosomal rearrangements. Genes Chromosomes Cancer 2004;41:339-52.
- Slape C, Aplan PD. The role of NUP98 gene fusions in hematologic malignancy. Leuk Lymphoma 2004;45:1341-50.
- Zhang H, Schneider J, Rosdahl I. Expression of p16, p27, p53, p73 and Nup88 proteins in matched primary and metastatic melanoma cells. Int J Oncol 2002;21:43-8.
- Agudo D, Gomez EF, Martinez AF, Nunez MJ, Pollan M, Schneider J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. Int J Cancer 2004;109:717-20.
- Gould VE, Orucevic A, Zentgraf H, Gattuso P, Martinez N, Alonso A. Nup88 (karyoporin) in human malignant neoplasms and dysplasias: correlations of immunostaining of tissue sections, cytologic smears, and immunoblot analysis. Hum Pathol 2002;33:536-44.
- Boer J, Bonten-Surtel J, Grosveld G. Overexpression of the nucleoporin CAN/NUP214 induces growth arrest, nucleocytoplasmic transport defects, and apoptosis. Mol Cell Biol 1998;18:1236-47.
