Anticancer activity of sodium caffeate and its mechanism1
Introduction
There have been an increasing number of anticancer phytochemicals identified in our daily diet. Some of the most promising and extensively investigated are those present in the cruciferous family of vegetables, alliums and tea. Phytochemicals should be considered as an inexpensive and readily applicable, acceptable and accessible approach to cancer control and management for general populations. This is particularly important considering the sluggish progress made in cancer treatment. It is still an urgent task to seek new anticancer drugs from natural resources in oncology pharmacology.
Cinnamic acid is one of the phytochemicals with potential chemopreventive effects in preventing carcinogenesis[1,2]. Cinnamamide, a natural compound containing the cinnamic acid structure, is a new antitumor agent that acts on matrix metalloproteinase, which has been demonstrated by previous work in our laboratory[3]. Caffeic acid (3,4-dihydroxycinnamic acid) is a polyphenol that is found in coffee, fruits, vegetables, grains and many others plants[4–7]. It is also particularly abundant in propolis beehives with 20%–25% content and has various pharmacological activities, such as antioxidant and antiviral effects[8]. The anticancer effect of caffeic acid, however, has not been reported up to now. Because caffeic acid is prone to air oxidation and is only slightly soluble in water, its stable sodium salt (sodium caffeate, SC) was prepared in our laboratory and used in the present study. Here we report the antitumor effect of SC both in vitro and in vivo.
Materials and methods
Reagents RPMI-1640 medium was purchased from Gibco BRL (Gaithersburg, Maryland, USA). Fetal calf serum (FCS) was purchased from Hyclone (Logan, Utah, USA). MTT, nonidet P-40(NP-40), phenylmethylsulfonyl fluoride(PMSF), aprotinin, ponceau S, Triton X-100, propidium iodide, Fluo-3, rhodamine 123, RNase A, proteinase K, Hoechst 33342 and other reagents were purchased from Sigma (StLouis, Missouri, USA). [H3]TdR was purchased from Chinese Atomic Energy Institutes (Beijing, China). Annexin V-FITC/PI apoptosis detection kit was purchased from BioVision company (Hannover, Germany). Mouse anti-Bcl-2 monoclonal antibody, mouse anti-caspase-3 monoclonal antibody and rabbit anti-bax polyclonal antibody were products of Calbiochem (San Diego, California, USA). Horseradish peroxidase-conjugated secondary anti-mouse antibody and anti-rabbit antibody were products of Santa Cruz Biotechnology, Inc(Santa Cruz, California,USA). Enhanced luminol reagent and oxidizing reagent were products of NEN Life Science Products (Boston, Massachusetts, USA). Dr Dan-qing SONG in Department of Chemistry, Institute of Medicinal Biotechnology (Beijing, China) synthesized the SC.
Cells and carcinoma Human oral cavity epidermis squamocellular carcinoma cell line (KB), human hepatocarcinoma cell line (BEL-7402) and human acute promyelocytic cell line (HL-60) were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China), and were grown routinely in RPMI-1640 supplemented with 10% heat-inactivated FCS. The medium was supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin and 2 mmol/L glutamine, and the cells were incubated in a humidified atmosphere, with 5% CO2 in air at 37 °C.
Mouse-transplanted hepatocarcinoma H22 and mouse colorectal cancer C26 cell lines were maintained by serial transplantation into mice in our laboratory. Kunming species mice were supplied by the Experimental Animal Center, Chinese Academy of Medical Sciences (Beijing, China).
Nucleoside transport assay[9] Briefly, cells in the logarithmic growth phase were harvested. The cell suspension was prepared with RPMI-1640 medium at 5×106 in 0.9 mL in each test tube. Different concentrations of SC in 0.1 mL RPMI-1640 were added and the tube was kept in a water bath at 37 °C for 5 min. Phosphate-buffered solution (PBS) was used as a control. RPMI-1640 0.1 mL (containing 3.7×104 Bq of [3H]TdR in medium free of serum) was added for 30 s and 5 mL ice-cold normal saline was added to terminate the reaction. The reaction was filtrated through a GF/B glass fiber filter (Whatman International, Maidstone, UK) under vacuum. The filters were washed with 0.2 mL of 1 mol/L NaOH and 0.5 mL ethanol, dried under vacuum and placed in scintillation vials containing 2 mL of dimethylbenzene with 0.4% PPO/0.01% POPOP. The cpm (counts per minute) were measured using an LS-9800 scintillometer (Beckman Instruments, Fullerton, California, USA).
MTT assay Briefly, cells in the logarithmic growth phase were harvested and seeded in 96-well plates (Costar, Cambridge, Massachusetts, USA) overnight. The test compound was added and cells were further incubated for 72 h. The viability of cells was determined using the MTT assay according to the method described by Carmichael et al [10].
Long-term clonogenicity Cell survival was tested using a clonogenic assay, as described by Valduga et al[11]. Briefly, cells in the logarithmic growth phase were harvested and 250 cells/mL of a single-cell suspension was prepared with medium. The cell suspension 200 µL was seeded in 96-well plates (50 cells/well) overnight, and the test compound was added. After 1 week of incubation at 37 °C in air with 5% CO2, colonies were counted.
Flow cytometry After appropriate treatment, cells were harvested by centrifugation and washed with PBS. The cells were fixed with ice-cold 75% ethanol for 18 h at 4 °C. The cell apoptosis was measured according to the protocol of Annexin V-FITC/PI apoptosis detection kit. Cell cycles change was measured by treatment of the fixed cell suspensions which were washed with PBS and stained with 80 µL of 50 µg/mL propidium iodide and 50 µg/mL RNase A for 30 min in the dark. Samples were run through an EPICS XL flow cytometer (Coulter, Miami, Florida, USA). Results are presented as the number of cells versus the amount of DNA as indicated by the intensity of fluorescence[12]. The results on flow cytometry represented the average of 3 individual experiments.
Western blotting assay The cells were lysed in lysis buffer at 4 °C with sonication. The lysates were centrifuged at 15 000×g for 15 min and the concentration of protein in each lysate was determined using Coomassie Brilliant Blue G-250. Loading buffer was added to each lysate, which was subsequently boiled for 3 min and then electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose and incubated with anti-Bcl-2, anti-caspase-3 or anti-Bax antibodies and then with peroxidase-conjugated secondary antibodies. Detection was carried out using an enhanced chemiluminescence agent[13,14]. The results on Western blot analysis represented the average of 3 individual experiments.
Intracellular Ca2+ and mitochondrial membrane potential (ΔΨ) Following appropriate treatment, cells were collected by centrifugation and incubated at 37 °C with Fluo-3 for 40 min and then with rhodamine 123 for 20 min. Cells were then washed 3 times with cold PBS, and the intracellular Ca2+ concentration and Δψ were measured by flow cytometry[15–17].
Tumor transplantation and drug administration H22 ascites were diluted to 7.5×105/mL suspension and 0.2 mL of the cell suspension was inoculated subcutaneously into the right axilla of each mouse of the Kunming species (weighing 20±2 g). A C26 tumor-cell suspension was prepared by gently suspending tumor tissue in normal saline (1 g of tumor tissue with 3 mL of normal saline) in a cold water bath and inoculated as above. After 24 h of tumor cell inoculation, SC was administered intraperitoneally for 10 d. Normal saline was used as a control. On the d 11 the mice were killed, and body weight and tumor tissue were weighed.
Statistical analysis The data are the mean values of at least 3 experiments and are expressed as mean±SD. The Student’s t-test was used to compare data. P<0.05 was considered to be statistically significant.
Results
Inhibition of nucleoside transport by SC SC inhibited nucleoside transport in the hepatocarcinoma BEL-7402 cell line with an IC50 of 1.02 mg/mL. However, the inhibiting effect on nucleoside transport was not very strong.
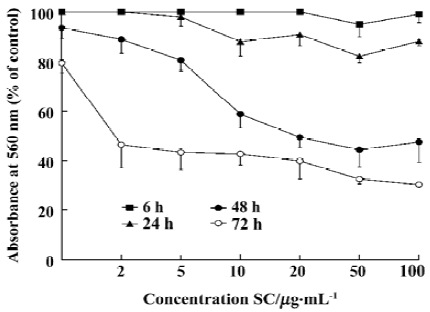
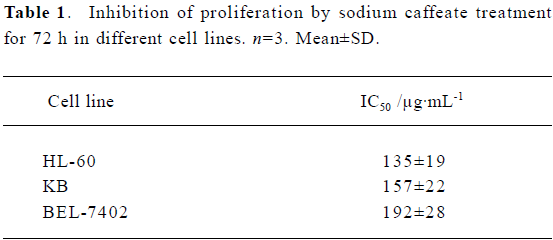
Inhibition of proliferation and induction of apoptosis by SC SC inhibited tumor-cell proliferation with an IC50 of between 100 µg/mL and 200 µg/mL (Table 1). Inhibition of BEL-7402 cells proliferaction by SC was dose-dependent and time-dependent (Figure 1).
Full table
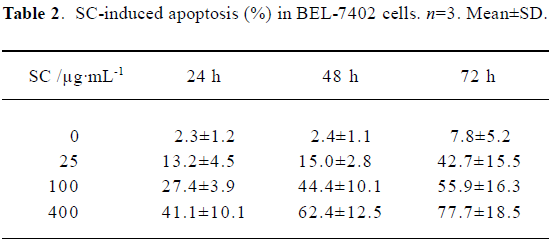
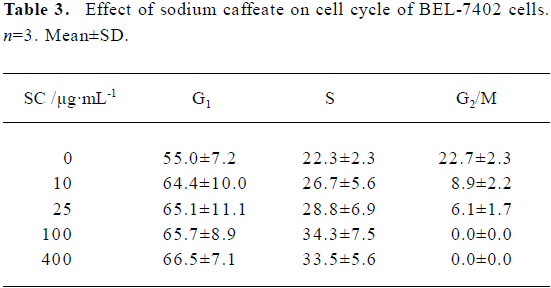
Flow cytometry showed that SC induced BEL-7402 cell apoptosis in a time- and dose-dependent manner (Table 2). After 24 h of treatment with SC, the cell cycle changed. The percentages of cells in S phase increased markedly while percentages of cells in G2/M phase decreased, which suggested the apoptosis was induced by arresting the cells in S phase (Table 3).
Full table
Full table
Inhibition of clonogenicity by SC SC inhibited cell clonogenicity with an IC50 between 0.5 µg/mL and 3 µg/mL (Table 4). SC was capable of inhibiting the different cell lines to different extents.
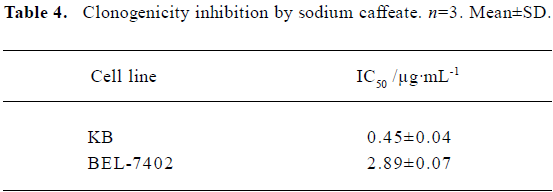
Full table
Effect of SC on transplanted tumor growth H22 and C26 were inoculated subcutaneously into mice. After SC administration for 10 d, tumor growth was inhibited in a dose-dependent manner. No significant difference in body weight was found between the groups, suggesting that SC does not show toxicity in vivo (Table 5 and 6).
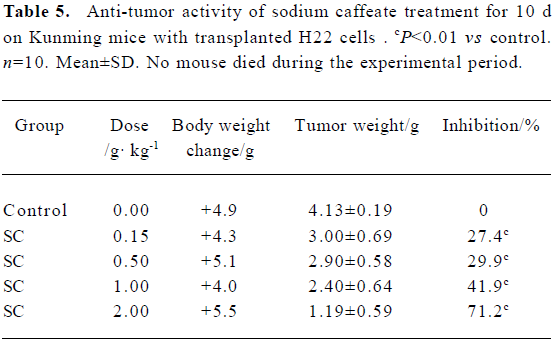
Full table
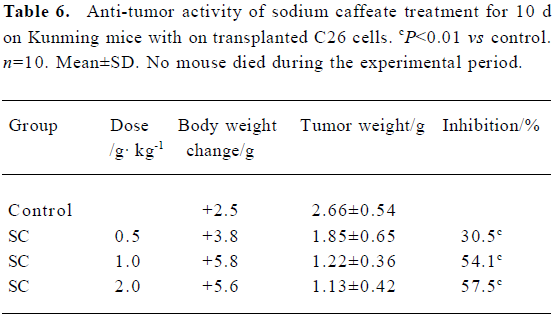
Full table
Effect of SC on expression of apoptosis-associated proteins The bands were scanned with light density. Activated caspase-3 and Bax expression were up-regulated after SC treatment, while Bcl-2 expression was down-regulated (Figure 2).
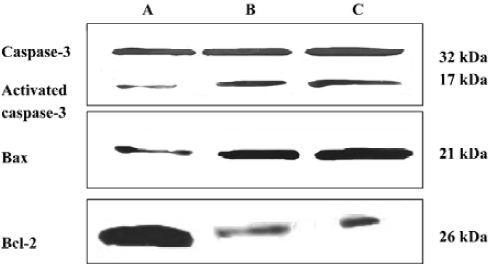
Effect of SC on intracellular Ca2+ and mitochondrial membrane potential After treatment with 10 μg/mL SC for 24 h, intracellular Ca2+ was increased 1.78-fold compared to the control. Δψ was decreased by 22.7% compared to the control. The results showed that SC increased intracellular Ca2+ levels and decreased Δψ.
Discussion
Most anti-metabolites in tumor chemotherapy inhibit nucleoside de novo synthesis but can not block nucleoside rescue in cancer cells. It is therefore important to control nucleoside rescue by inhibiting nucleoside transport. Previous work found that dipyridamole enhanced the anticancer effect of acivicin by inhibiting nucleoside transport[9].The present study demonstrated that SC was a new member of the nucleoside transport inhibitor family.
Caffeic acid is an active phytophenol that has been found to inhibit rat glutathione-S-transferase isoenzymes both in vitro and in vivo[18]. A large number of population-based studies have found that consumption of wholegrains, vegetables and fruits abundant in caffeic acid reduces the risk of cancer[19−21]. The aqueous extract of Salvia miltiorrhiza, a traditional Chinese herb containing caffeic acid was found to strongly inhibit the proliferation of human hepatoma HepG2 cells. It was also observed that its crude extract caused apoptotic cell death[22]. Salvianolic acid A, a caffeic acid trimer, showed synergistic effects in combination with other antitumor agents. Further, salvianolic acid A could increase the antitumor effects of 5-flurouracil without increasing its toxicity in an animal study [2]. However, no report has been published on the anticancer effects of caffeic acid either in vitro or in vivo. We are the first to report that the sodium salt of caffeic acid inhibits proliferation of cancer cells, with IC50 between 100 µg/mL and 200 µg/mL. Further, we showed that it induced the apoptotic cell death and changed cell-cycle distribution by arresting cells in S phase. The in vivo study showed that SC inhibited the tumor growth of transplanted H22 and C26 cells in mice with an inhibition rate of 42%–54% when treated with 1 g/kg SC for 10 d.
Preliminary studies on the anticancer mechanism of SC demonstrated that after treatment with SC, Bcl-2 expression was down-regulated and mitochondrial membrane permeabi-lity was changed. The mitochondrial permeability transition pore was opened and the mitochondrial membrane potential was broken up. The mitochondrion was swelled and in α state of hyperosmosis before apoptosis was induced. Meanwhile cytochrome c was released, caspase-3 was activated in the presence of Apaf-1 and caspase-9, and apoptosis was induced[23–27].
References
- Liu L, Hudgins WR, Shack S, Yin MQ, Samid D. Cinnamic acid: a natural product with potential use in cancer intervention. Int J Cancer 1995;62:245-50.
- Jiang RW, Lau KM, Hon PM, Mak TC, Woo KS, Fung KP. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem 2005;12:237-46.
- Jiang XF, Zhen YS. Cinnamamide, an antitumor agent with low cytotoxicity acting on matrix metalloproteinase. Anticancer Drugs 2000;11:49-54.
- Radtke J, Linseisen J, Wolfram G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Ernahrungswiss 1998;37:190-7.
- Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature 2000;405:903-4.
- Mattila P, Kumpulainen J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J Agric Food Chem 2002;50:3660-7.
- Bryngelsson S, Dimberg LH, Kamal-Eldin A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L). J Agric Food Chem 2002;50:1890-6.
- Greenaway W, Scaysbrook T, Whatley FR. The analysis of bud exudate of and of propolis by gas chromatography-mass spectrometry. Proc R Scot Lond 1987; B232: 249–72.
- Zhen YS, Liu MS, Weber G. Effects of acivicin and dipyridamole on hepatoma 3924A cells. Cancer Res 1983;43:1616-9.
- Carmichael J, Degraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987;47:936-42.
- Valduga G, Reddi E, Garbisa S, Jori G. Photosensitization of cells with different metastatic potentials by liposome-delivered Zn-(II)-pathalocyanine. Int J Cancer 1998;75:412-7.
- Chiao C, Carothers AM, Grunbergers D, Solomon G, Preston GA, Barrett JC. Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res 1995;55:3576-83.
- Hu ZB, Minden MD, McCulloch EA. Post-transcriptional regulation of bcl-2 in acute myleoblastic leukemia: significance for response to chemotherapy. Leukemia 1996;10:410-6.
- Xu F, Zhen YS. (-)-Epigallocatechin-3-gallate enhances anti-tumor effects of cytosine arabinoside on HL-60 cells. Acta Pharmacol Sin 2003;24:163-8.
- Kluck RM, McDougall CA, Harmon BV, Halliday JW. Calcium chelators induce apoptosis: evidence that raised intracellular ionized calcium is not essential for apoptosis. Biochim Biophys Acta 1994;1223:247-54.
- Tatton WG, Olanow CW. Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta 1999;1410:195-213.
- Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low to high conductance state. Biochim Biophys Acta 1998;1366:33-50.
- Ploemen JH, van Ommen B, de Haan A, Schefferlie JG, van Bladeren PJ. In vitro and in vivo reversible and irreversible inhibition of rat glutathione-S-transferase isoenzymes by caffeic acid and its 2-S-glutathionyl conjugate. Food Chem Toxicol 1993;31:475-82.
- Shahrzad S, Bitsch I. Determination of some pharmacologically active phenolic acids in juices by high-performance liquid chromatography. J Chromatogr A 1996;741:223-31.
- Slavin J, Jacobs D, Marquart L. Whole-grain consumption and chronic disease: protective mechanisms. Nutr Cancer 1997;27:14-21.
- Surh YJ. Transcription factors in the cellular signaling network as prime targets of chemopreventive phytochemicals. Cancer Treat Res 2004;36:275-86.
- Liu J, Shen HM, Ong CN. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Lett 2000;153:85-93.
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997;275:1129-32.
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 1998;281:1322-6.
- Thornberry NA, Lazebnik Y. Caspase: enemies within. Science 1998;281:1312-6.
- Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309-12.
- Wallace DC. Mitochondrial disease in man and mouse. Science 1999;283:1482-8.