Synergistic antitumoral activity and induction of apoptosis by novel pan Bcl-2 proteins inhibitor apogossypolone with adriamycin in human hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and is the fifth most common cancer in the world, resulting in more than 660 000 deaths annually worldwide[1,2]. Despite its rising incidence, the overall prognosis of HCC is poor, as systemic chemotherapy is of low efficacy[3,4]. The most widely-used treatment is adriamycin (ADM), either alone or in combination with other drugs. However, the response rate of ADM in a phase III clinical trials was only 10.5% when used alone and 20.9% for PIAF (cisplatin/interferon α-2b/ADM/fluorouracil) combination therapy[5], but chemotherapy usually causes cytotoxicity with poor selection. Side-effects were frequently observed with each of the treatments. On the contrary, the newly-developed target therapies focus on the tumor-specific proteins or kinases that are overexpressed in cancer cells or affect the development of tumors. Those agents have shown promising effects on liver cancer in clinical trials, such as the RAF/MAP kinase kinase (MEK)/extracellular signal-regulated protein kinase (ERK) pathway blocker Nexavar[6], which is a multikinase inhibitor approved by the FDA for HCC therapy. Lifespan was prolonged by 44% in comparison with the placebo group according the data of a phase III clinical trial[7].
It is well-established that the overexpression of Bcl-2 family proteins plays an important role in tumor progression and drug resistance[8–10], and is associated with unfavorable outcomes. The Bcl-2/Bcl-XL protein has a Bcl-2 homology domain 3 (BH3)-binding groove that can be combined with many pro-apoptotic factors, such as Bad and Bak or BH3-only proteins, such as Bid, Bim, Noxa, and Puma and forms heterodimers, thereby inhibiting their function and inducing apoptosis. Targeting the BH3 domain of anti-apoptotic Bcl-2 members using non-peptide small-molecule inhibitors is a new and exciting therapeutic strategy[11]. For example, ABT-737[12], which has high affinity to Bcl-2 and Bcl-XL, has shown potent inhibitory effects on the proliferation of tumor cells. Gossypol is another example of a potent inhibitor of Bcl-2 family proteins. It is a polyphenolic aldehyde occurring naturally in cottonseed. It has been used and extensively investigated as a male oral contraceptive agent and was recently discovered as a potent pan inhibitor of Bcl-2 family proteins[13,14]. Gossypol has demonstrated beneficial anti-tumoral effect on a variety of cancer cells[15–19]. Bcl-2-directed treatment targets cancer cells specifically over-expressing Bcl-2 proteins and is less toxic than traditional chemotherapy. Therefore, employing small molecules targeting Bcl-2 has become a popular approach for targeted cancer therapy.
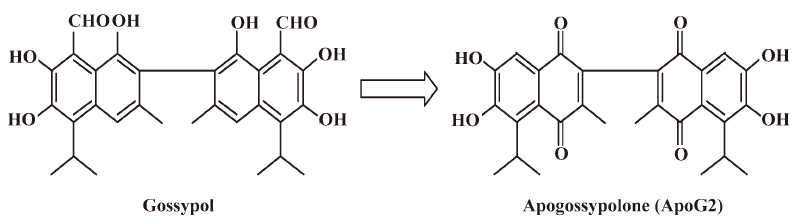
To overcome the potential non-specific reactivity related to the 2 aldehyde groups in gossypol[20], apogossypolone (ApoG2) was designed and synthesized, and 2 aldehyde groups of gossypol were completely removed (Figure 1). The Ki values of ApoG2 binding with Bcl-2, Mcl-1, and Bcl-XL were 35, 25, and 660 nmol[21], respectively. Studies on ApoG2 for the treatment of pancreatic cancer, prostate cancer, leukemia, and lymphoma have been reported[21–24]. The treatment of human pancreatic cell line BxPC-3 with curcumin sensitized ApoG2-induced cell killing. Bcl-XL and Bcl-2 expression levels were downregulated, along with the inactivation of NF-κB in combination with curcumin[22]. In addition, a higher apoptotic index (94%) of ApoG2 was observed in combination with gemcitabine in BxPC-3 cells[23]. An in vivo study showed that ApoG2 could significantly increase the lifespan of lymphoma-bearing SCID mice by at least 42%[23]. Those results indicated that ApoG2 was a potential pan Bcl-2 family protein inhibitor, targeting Bcl-2, Mcl-1, and Bcl-XL, and inducing apoptosis in cancer cells alone or in combination therapy.
As cancer cell-specific targets are not the only part of disease etiology, treatments combining targeted and conventional drugs may have a synergistic effect. In the present study, we investigated the in vitro and in vivo activities of ApoG2, as well as its activity in combination with chemotherapy agent ADM in HCC.
Materials and methods
Cells The human liver cancer cell lines SMMC-7721, Bel-7402, and QGY-7703 were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). HepG2 was purchased from American Type Culture Collection (Manassas, VA, USA). All cells were cultured in RPMI-1640 (GIBCO, Gaithersburg, MD, USA) with 10% fetal bovine serum (GIBCO), 1% L-glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin. The cells were incubated at 37 °C in a humidified incubator with 5% CO2.
Reagents ApoG2 were synthesized by Ascenta Therapeutics (Malvern, PA, USA). For the in vivo study, ApoG2 was suspended in 0.5% carboxymethylcellulose (CMC) solution and administered by oral gavage. ADM was purchased from Shanghai Richem International (Shanghai, China). For the intravenous treatment study, it was prepared in 0.9% sterile saline solution.
For the in vitro study, ApoG2 and ADM were dissolved in 100% DMSO and made to a 10 mmol/L store solution on the day of treatment. The working solution was prepared by dilution of the store solution with the culture medium.
Rabbit anti-Bcl-XL (N
WST assay The inhibition of cell growth was determined by WST assay using cell counting kit-8 (CCK-8) from Dojindo Molecular Technologies (Kumamoto, Japan). The cells were seeded onto 96-well plates and treated with different concentrations of ApoG2 (0.1−80 μmol/L) alone for 72 h. The combinable effect of ApoG2 and ADM was assayed using the 2 agents separately (ApoG2 at 1.97–10 μmol/L or ADM at 0.11–0.6 μmol/L) or in combination and at a fixed drug dilution ratio. The cells were also treated for 72 h. Three repeat wells per treatment were required. After adding 10 μL CCK-8 to each well, the plates were incubated for 2 h. Absorbance at 450 nm was then measured using the Spectra Max 190 (Molecular Devices) microplate reader. Results were presented as the IC50 of ApoG2, dose effect, and combination index (CI) values of ED50, ED70, and ED90 for the combined treatment of ApoG2 and ADM.
The IC50 was calculated with GraphPad Prism 4 software (Golden software, Golden, Colorado, USA). Drug interaction was evaluated by using CalcuSyn software (Biosoft, Ferguson, MO, USA). Drug combination studies were analyzed with the CI; CI values less than 1.00 were considered as showing synergetic effects.
4’,6-Diamidino-2-phenylindole assays and flow cytometric analysis of apoptosis and cell cycle in cultured cells Cell apoptosis and the nuclei changes were estimated by 4´,6-diamidino-2-phenylindole (DAPI) staining. The cells were seeded in 6-well plates and treated with ApoG2 (5, 10, or 15 μmol/L), ADM (1 μmol/L), and ApoG2 (10 μmol/L) in combination with ADM at (1 μmol/L). The cells in the control group were treated with the culture medium. The cells were collected 48 h after treatment and fixed with 4% formaldehyde; cell nuclei were stained with DAPI (Sigma, St Louis, MO, USA). The stained cells were collected and the sections were made. The changes in the nuclei were observed under a fluorescent microscope with a 360 nm filter.
DNA contents were analyzed by flow cytometry. The cells were fixed by 70% ethanol at 4 °C for at least 1 h. After washing with phosphate-buffered solution (PBS), the cells were incubated in RNase A/PBS (100 mg/mL) at 37 °C for 30 min. Intracellular DNA was labeled with propidium iodide (PI) (50 mg/mL) and analyzed with a FACSCalibur fluorescent-activated cell sorter (FACS; Becton Dickinson, Franklin Lakes, NJ, USA). The cell cycle profile and apoptosis was obtained by analyzing 10 000 cells.
Western blotting To determine changes in the protein levels, the cells were seeded onto 10 cm dishes and treated with ApoG2 at 5, 10, or 15 μmol/L, ADM at 1 μmol/L, and ApoG2 at 10 μmol/L in combination with ADM at 1 μmol/L. The control cells were treated with the culture medium. Floating and adherent cells were collected 48 h after treatment. For the P53 assay, the cells were collected 24 and 48 h after treatment. The cells were lysed using lysate buffer (Cell Signaling Technology, USA). The protein concentration of the supernatant was detected using the bicinchoninic Acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Haimen, China). The cell lysates were loaded to the 12% SDS gels and subsequently transferred to Hybond P membranes (GE Healthcare, USA). The first antibodies were incubated with the membranes at 4 °C overnight. The peroxidase-conjugated secondary antibodies were incubated with the membranes at room temperature for 2 h. Protein bonds were detected by ECL detection kit (GE Healthcare, USA).
Detecting the activation of caspases-9 and -3 The activities of caspases-9 and -3 of the cell lysates was measured using the caspase-9 and caspase-3 colorimetric assay kit (Nanjing KeyGen Biotechnology, Nanjing, China), according to the manufacturer’s instructions. Alternatively, the cells were treated and collected as described in the Western blot assay. The protein concentration of the cell lysate was measured by BCA assay. The cell lysate was incubated with the chromophore-coupled substrates of caspases-9 and -3 at 37 °C for 4 h. The chromophore liberated from the substrate was measured using a plate reader at 405 nm. The activities were shown as the ratio of the optical density (OD) agent-treated sample/OD negative control sample.
Antitumoral effect of ApoG2 alone or in combination with ADM in animal models (nude mouse xenograft) Female 6-week-old Balb/c nu/nu mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China); 5×106 SMMC-7721 cells suspended in 0.2 mL PBS were inoculated sc to the right oxtor of each nude mice. Volumes of the tumor were estimated as V=LW2/2, where L and W stand for tumor length and width, respectively. Mice with tumors at 50–150 mm3 were randomized into treatment groups (6 mice per group in the treatment groups, and 10 mice in the vehicle control group) and commenced treatment.
The mice were treated with ApoG2 at 100 and 200 mg/kg for 28 d by intragastric injection alone or in combination with ADM at 5.5 mg/kg once a week intravenously for 4 weeks. The positive control group was treated with ADM at 5.5 mg/kg once a week intravenously for 4 weeks. The vehicle control group was dosed ig daily with 0.5% CMC for 4 weeks. The individual relative tumor volume (RTV) was calculated as follows: RTV=Vt/V0, where Vt is the volume on each day of measurement, and V0 is the volume on the initial day of treatment. The therapeutic effect of the compound is expressed with the relative tumor proliferation rate (T/C). The calculation formula was: T/C=mean RTV of the treated group/mean RTV of the control group. Treatments producing>20% lethality and/or 20% net body weight loss were considered toxic.
Tissue microscopy In another pilot study, when the tumor size reached 150−200 mm3, 3 mice per group were treated with ApoG2 along (200 and 400 mg/kg, ig, daily) for 7 d, ADM at 10 mg/kg once intravenously on the first day, or ApoG2 in combination with ADM at 10 mg/kg. The vehicle control group was ig dosed daily with 0.5% CMC for 7 d. The mice were euthanized 2 h after the last treatment. Tumors and some tissues were removed from the animals and fixed in 10% formalin solution immediately. Tissues were paraffin embedded, and 0.5 μm-thick sections were prepared. The tumor, liver, lung, heart, kidney, spleen, and gastric tissues were stained with hematoxylin–eosin.
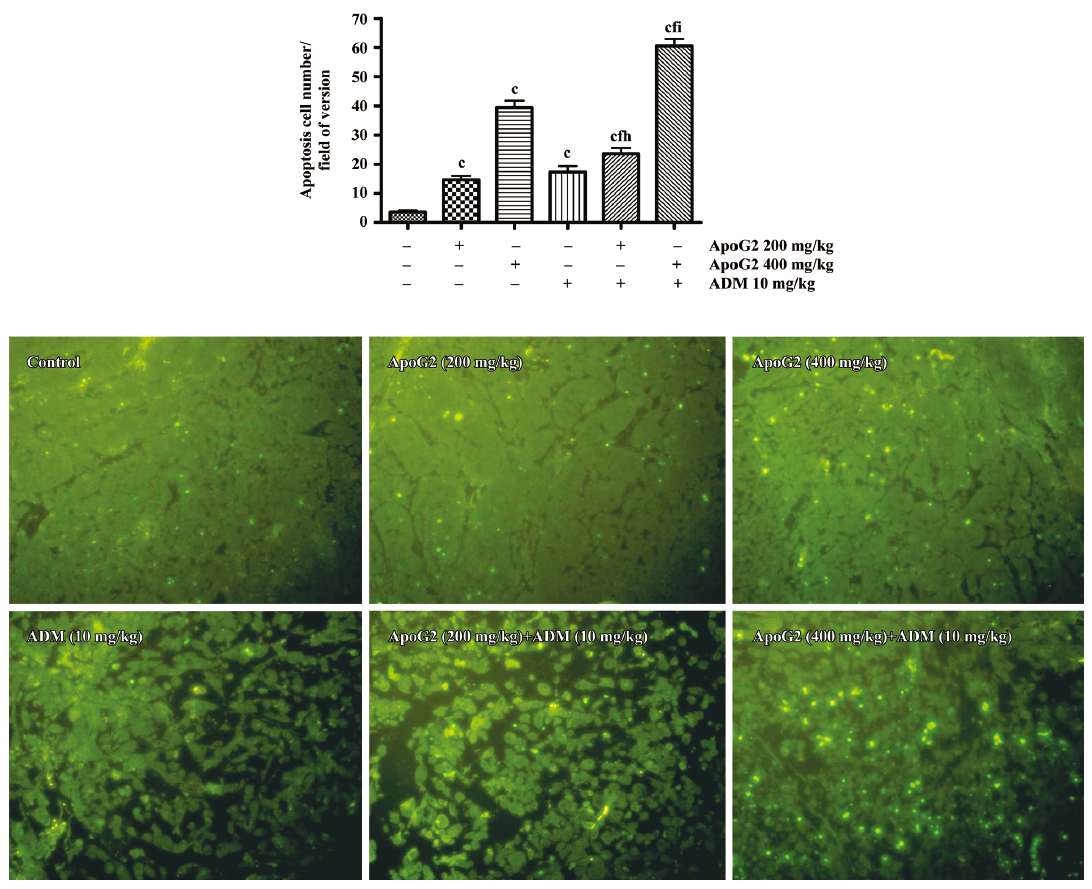
Terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP nick-end labeling assay To examine apoptosis in the specimens, sections were stained by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the fluorescein FragEL DNA fragmentation detection kit (Calbiochem, USA), according to the manufacturer’s instructions. Briefly, the slides were dewaxed in xylene and 70%–100% ethanol at room temperature as routine protocol. The slides were rinsed with 1×TBS and the entire specimen was incubated with 100 μL of 20 μg/mL proteinase K at room temperature for 20 min. The slides were rinsed with 1×TBS and the entire specimen were covered with 1 μg/μL DNase I in 1×TBS/1 mmol/L MgSO4. The slides were incubated at room temperature for 20 min. After washing with 1×TBS, the specimens were incubated with 100 μL of 1×terminal deoxynucleotidyl transferase (TdT) equilibration buffer at room temperature for 10–30 min. The 1×equilibration buffer was carefully blotted from the specimen and 60 μL TdT labeling reaction mixture was immediately applied onto each specimen. The slides were placed in a humidified chamber and incubated at 37 °C for 1–1.5 h. After washing with 1×TBS, the slides were mounted with a glass coverslip and the edges were sealed. The labeled nuclei in the slides were analyzed by using a standard fluorescent filter at 465–495 nm. Ten high-power fields were examined for each sample, and the number of cells stained positive in each field was determined. Values were then averaged to provide an apoptotic number for each tumor specimen, and an average of 3 tumors was determined for each tumor group.
Statistical analysis Statistical evaluation was performed using two-tailed t-test. P-values less than 0.05 were considered statistically significant.
Results
Evaluation of the combined growth inhibitory effects of ApoG2 and ADM in cultured human liver cancer cells The in vitro cytotoxicity of ApoG2 on the 4 HCC cell lines were determined by WST assay (Figure 2). The 4 cell lines had a high expression level of Bcl-2 family proteins. The IC50 value of ApoG2 for the SMMC-7721, Bel-7402, QGY-7703, and HepG2 cell lines were 17.28±4.71, 21.61±5.25, 17.36±4.30, and 30.63±3.83 μmol/L, respectively (Figure 2A). The most sensitive cell lines were SMMC-7721 and QGY-7703.
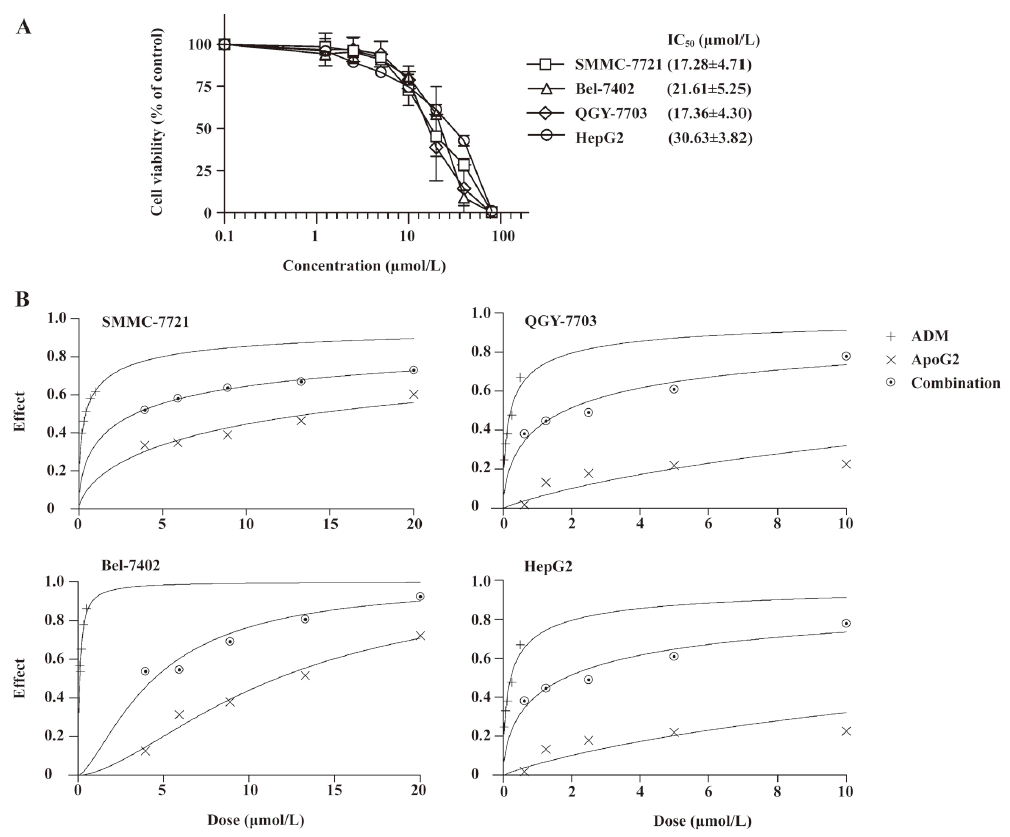
Increased cytotoxicity was observed when ApoG2 was used in combination with ADM in the HepG2, SMMC-7721, and QGY-7703 cell lines, calculated by the CalcuSyn method, but not in the Bel-7402 cell line. The data demonstrated that the CI plots of interactions between ApoG2 and ADM in HepG2, SMMC-7721, and QGY-7703 cells after 72 h treatment were less than 1.0 (Figure 2B, Table 1), which indicated synergy between two agents. As HepG2 has poor tumorigenicity in nude mice, and SMMC-7721 was the most sensitive cell line to ApoG2 in our studies and readily formed progressive tumors in nude mouse xenograft models, further detailed investigations were carried out in this cell line.
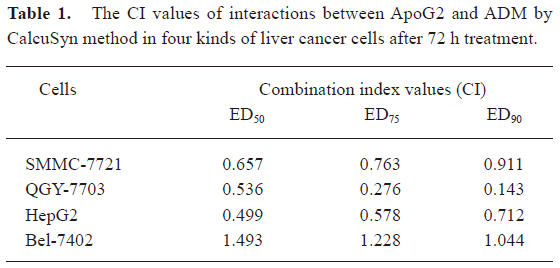
Full table
ApoG2 alone or in combination with ADM induced apoptosis of SMMC-7721 and influenced the expression of apoptosis-related proteins ApoG2 in combination with ADM resulted in increased apoptosis in SMMC-7721 cells. The results of the DAPI staining indicated that ApoG2 induced apoptosis alone or in combination with ADM (Figure 3A). The data of the FACS analysis demonstrated that ApoG2 at 5, 10, and 15 μmol/L increased the counting apoptosis rate by 3.87%, 12.92%, and 30.76%, respectively. The apoptosis rate for ADM at 1 μmol/L was 3.89%, and an enhanced apoptosis rate of 18.67% was detected for ApoG2 at 10 μmol/L with ADM at 1 μmol/L in the cultured cells (Figure 3C). The cell cycle analysis demonstrated that ApoG2 also induced cell arrest at the G0/G1 phase, whereas ADM caused S-phase arrest in SMMC-7721 cells, both alone and in combination, compared with 10 μmol/L ApoG2 (Figure 3B).
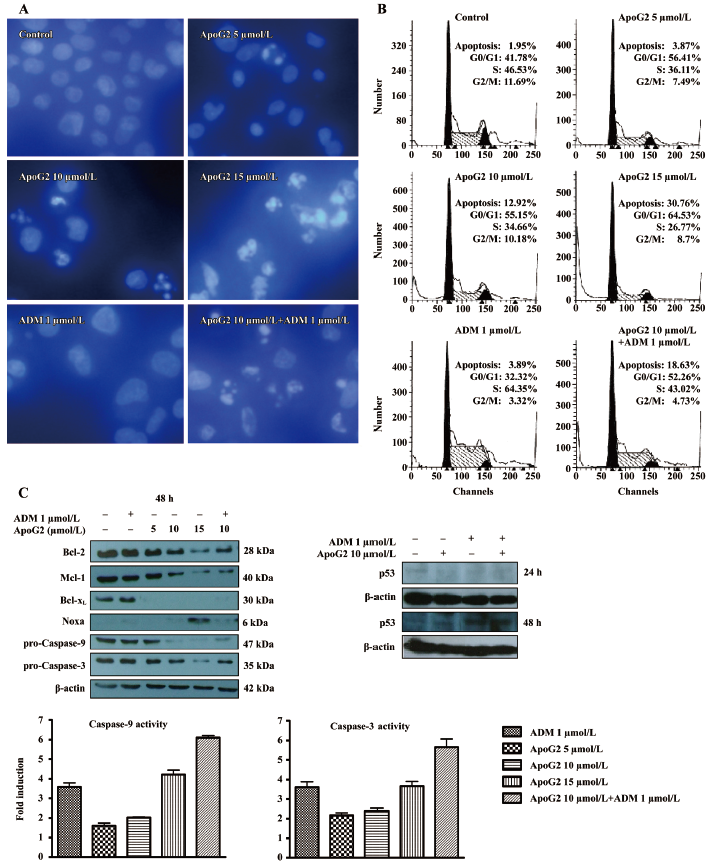
The expressions of anti-apoptotic proteins Bcl-2 and Mcl-1 were reduced by ApoG2 in a dose-dependent manner, and the expression of Bcl-XL was completely inhibited by an even lower concentration of 5 μmol/L in this study. The BH3-only pro-apoptotic protein Noxa was increased by ApoG2 at 15 μmol/L. ApoG2 reduced full-form caspases-9 and -3, and the caspase activity assay indicated that the activities of caspases-9 and -3 increased in a dose-dependent manner. The results demonstrated that ApoG2 could induce apoptosis through the Bcl-2 pathway and activate a caspase cascade reaction (Figure 3C).
There appeared to be no difference in all of the protein expressions between 10 μmol/L ApoG2 alone and the combination of ApoG2 at 10 μmol/L with ADM, according to the Western blot analysis. Although ADM alone did not affect the expression of Bcl-2 family members, it could promote the activities of caspases-9 and -3 and induce apoptosis for SMMC-7721. Enhanced activities of caspases-9 and -3 were observed in combination treatment. As p53 plays an important role in chemotherapy, further studies examining p53 should show that ADM increases the expression of p53 both alone and in combination with ApoG2 after treatment for 48 h (Figure 3C). The treatment of SMMC-7721 cells with concentrations up to 10 µmol/L ApoG2 for 48 h did not produce significant changes in the levels of p53 in this assay.
Growth inhibition in the SMMC-7721 xenograft model In the liver cancer SMMC-7721 xenograft model, ApoG2 alone at a dose of 100 or 200 mg/kg (ig, once daily for 28 d) resulted in modest tumor growth inhibition with T/C values of 0.688 and 0.676, respectively. The T/C value for intravenous ADM at 5.5 mg/kg (once a week for 4 weeks) was 0.853, indicating limited or no antitumoral activity. An enhanced effect of ADM was observed when combined with ApoG2, with T/C values of 0.456 (P<0.05) and 0.323 (P<0.01) for 100 mg/kg ApoG2 with ADM or 200 mg/kg ApoG2 with ADM, respectively.
Significant body weight loss was not observed in the ApoG2-alone treatment groups compared with the control group during the experiment. However, ADM caused significant weight loss whether it was used alone or not (P<0.05), especially during the second and third weeks of treatment. Nevertheless, the weight changes were recovered after the cessation of treatment.
Figure 4C shows the damaged cells in combination therapy-treated tumor tissues. Compared with the control or single agent-dosing groups, there were spaces between the cells (cells were necrotic) and pyknotic nuclei in the slides of the tumor tissues of the combination groups. However, there were no significant lesions in the normal liver or other tissues, even with a high dose of ApoG2 (Figure 4D).
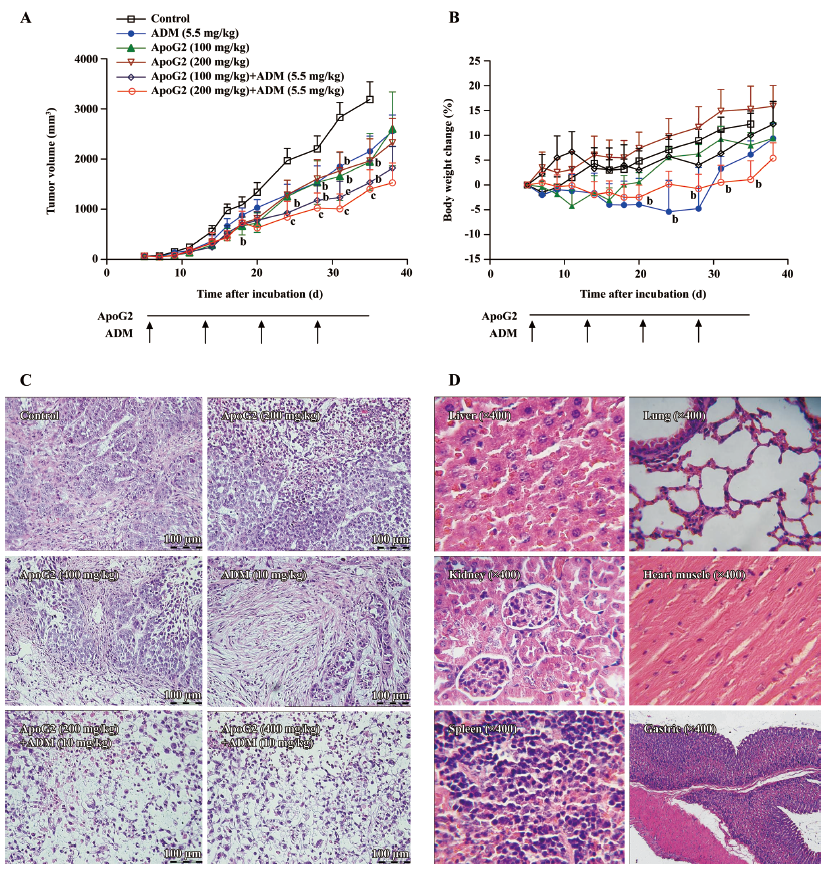
The results demonstrated that ApoG2 had low toxicity to normal tissues and demonstrated excellent therapeutic potential for human liver cancer either alone or in a combinable regimen.
Induction of apoptosis in tumor tissues The mice were treated with ApoG2 alone (200 or 400 mg/kg, ig, daily) for 7 d, or with ADM at 10 mg/kg once intravenously on the first day, or with ApoG2 in combination with ADM at 10 mg/kg. The vehicle control group was dosed ig daily with 0.5% CMC for 7 d. The mice were killed 2 h after the last treatment. The apoptotic cells in the tumor sections were detected by TUNEL assay. In this assay, TdT bound to the exposed 3´-OH ends of DNA fragments generated in response to apoptotic signals and catalyzed the addition of fluorescein-labeled deoxynucleotides. The green fluorescent staining in the tumor sections represented apoptotic cells (Figure 5). The number of apoptotic cells in the ApoG2 alone group at 400 mg/kg was more than that of the ApoG2 alone group at 200 mg/kg. ApoG2 at 400 mg/kg with ADM at 10 mg/kg significantly induced more apoptotic cells in comparison with those treated with either ApoG2 or ADM alone in SMMC-7721 tumor tissues.
Discussion
ApoG2 is a potent, small molecule inhibitor of Bcl-2, Bcl-XL, and Mcl-1 by binding to the BH3 binding pocket. The Bcl-2 family members all contain a BH3 domain, and it has been proposed that gossypol and its derivatives, acting as non-selective BH3 mimetic polygon, may bind to the BH3-binding pocket of various Bcl-2 family members. This binding activates Bak or Bax directly and promotes cell death, also simultaneously inhibiting anti-apoptotic members[25]. Our study demonstrated that ApoG2 could dose dependently alter the expression of Bcl-2 family members, downregulating Bcl-2, Mcl-1 and Bcl-XL, and up-regulating the expression of pro-apoptotic proteins, such as Noxa.
With the binding of ApoG2 to Bcl-2 family proteins, it would be expected that ApoG2 could lead to the activation of downstream apoptotic proteins. In the present study, we show that ApoG2 can activate caspase-9, which in turn activates caspase-3. Caspase-3 is one of the key executioners of apoptosis. Biochemical and genetic evidence indicates that Bcl-2 prevents apoptosis at the point of caspase-3 activation[26]. Here, its changes corresponded with the results of induced apoptosis in SMMC-7721 cells.
These findings demonstrate that ApoG2 can activate the Bcl-2 apoptotic pathway in vitro. The exact mechanism of action of ApoG2 is still not clear. The proposed mechanisms would be that ApoG2 binds to Bcl-2 family members and its association interferes with BH3-only pro-apoptotic proteins, thus pro-apoptotic proteins participate in the apoptotic response. The results of the cell cycle assay also indicated that ApoG2 could induce cell cycle arrest at the G0/G1 phase in SMMC-7721 cells. Studies of (-)-gossypol show that it induces G0/G1 arrest in colon cancer and lymphoma cells[27,28]. As a derivative of gossypol without two aldehyde groups, ApoG2 could inhibit the proliferation of SMMC-7721 through cell cycle arrest and the induction of apoptosis.
The activation of P53, which promotes apoptosis of tumor cells, is considered to be a key mechanism of action of antitumoral drugs, including ADM[29,30]. In this experiment, although ADM did not affect Bcl-2 family proteins of SMMC-7721, other studies on P53 showed that ADM could promote P53 expression. The results indicated that the promotion of P53 expression would be a possible cause for the induction of apoptosis by ADM in SMMC-7721 cells. ADM could inhibit biosynthesis of nucleic acid in tumor cells and cause cell toxicity in all the cell cycle phases. In our study, ADM at 1 μmol/L induced S-phase arrest to SMMC-7721 cells. Nevertheless, based on the data presented in this study, apoptosis and cytotoxicity induced by combination therapy of ApoG2 with ADM is more significant than with either agent alone.
The in vivo data found that the effect of ApoG2 alone was stronger than that of ADM alone, and that ApoG2 alone at an oral dose of 100 or 200 mg/kg, once daily for 28 d, resulted in modest tumor growth inhibition and T/C values of 0.688 and 0.676, respectively, in the liver cancer SMMC-7721 xenograft model. The T/C value for intravenous ADM at 5.5 mg/kg (once a week for 4 weeks) was 0.853. More importantly, there was no body weight loss in the animals treated with ApoG2 with all doses, indicating no toxicity to the animals at the dosages administered. The maximal tolerated dose (MTD) of ApoG2 in the mice by oral gavage was more than 2000 mg/kg in our separate studies. Furthermore, histopathological examinations indicated that no lesions were found in the normal tissues of major organs in mice after continuing treatment with ApoG2 at 400 mg/kg for 7 d. The combination treatment did not result in greater toxicity to the animals. When combined with ADM, an enhanced effect of ApoG2 was observed, with T/C values of 0.456 (P<0.005) and 0.323 (P<0.001) for the 100 mg/kg plus ADM and 200 mg/kg plus ADM groups, respectively. These results indicate that ApoG2 would be a safe and effective agent for anti-liver cancer therapy either alone or in combination therapy.
In summary, the data demonstrated that ApoG2 can promote apoptosis through the Bcl-2 pathway by upregulating the BH3-only pro-apoptotic protein Noxa and downregulating Bcl-2, Mcl-1, and Bcl-XL in SMMC-7721 cells. ApoG2 could have great therapeutic potential as an effective new therapy for HCC when used with traditional chemotherapy ADM.
References
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17.
- World Health Organization. Media centre factsheets. Available from URL: www.who.int/mediacentre/factsheets/ fs297/en
- Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol 1997;7:387-96.
- Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol 1997;8:117-36.
- Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 2005;97:1532-8.
- Liu L, Cao YC, Chen C, Zhang XM, McNabola A, Wilkie D, . Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006; 66: 11 851−8.
- Llovet J, Ricci S, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S, . Randomized phase III trial of sorafenib versus placebo in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition); 2007; 25 Suppl: LBA1.
- Lacronique V, Minon A, Fabre M, Viollet B, Rouquet N, Molina T, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 1996;2:80-6.
- Chuna EY, Lee KY. Bcl-2 and Bcl-xL are important for the induction of paclitaxel resistance in human hepatocellular carcinoma cells. Biochem Biophys Res Commun 2004;315:771-9.
- Takahashi M, Saito H, Atsukawa K, Ebinuma H, Okuyama T, Ishii H. Bcl-2 prevents doxorubicin-induced apoptosis of human liver cancer cells. Hepatol Res 2003;25:192-201.
- Wang S, Yang D, Lippman ME. Targeting Bcl-2 and Bcl-XL with nonpeptidic small-molecule antagonists. Semin Oncol 2003;30:133-42.
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005;435:677-81.
- Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ 2006;13:1419-21.
- Oliver CL, Bauer JA, Wolter KG, Ubell ML, Narayan A, O'Connell KM, et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res 2004;10:7757-63.
- Lei XB, Chen YY, Du GH, Yu WY, Wang XH, Qu H, et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. FASEB 2006;20:E1510-9.
- Mohammad RM, Wang SM, Aboukameel A, Chen B, Wu XH, Chen JY, et al. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL [(-)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther 2005;4:13-21.
- Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee J S, et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther 2005;4:1096-104.
- Huang YW, Wang LS, Chang HL, Ye WP, Dowd MK, Wan PJ, et al. Molecular mechanisms of (-)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Res 2006;26:1925-34.
- Wolter KG, Wang SJ, Henson BS, Wang SM, Griffith KA, Kumary B, et al. (-)-Gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia 2006;8:163-72.
- Stein RC, Joseph AE, Matlin SA, Cunningham DC, Ford HT, Coombes RC. A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother Pharmacol 1992;30:480-2.
- Zhang Y, Ling X, Zhang XW, Lin YQ, Min P, Stoudemire J, . A novel pan inhibitor of Bcl-2 and Mcl-1 apogossypolone (ApoG2) with superior stability and improved activity against human leukemia and lymphoma cells. Proceedings of 98th AACR Annual Meeting; 14–18 Apr 2007; Los Angeles, CA, USA.
- Thoutreddy S, Mohammad RM, Ali S, Wang SM, Sarkar H. Curcumin sensitizes pancreatic cancer cells to ApoG2 induced killing. Pancreas 2006;33:502. [Abstract].
- Arnold AA, Aboukameel A, Chen JY, Yang DJ, Wang SM, Al-Katib A, et al. Preclinical studies of apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-XL and Mcl-1 proteins in follicular small cleaved cell lymphoma model. Mol Cancer 2008;7:20.
- Choi M, Wang SM, Banerjee S, Sarkar F, Mohammad R. Bcl-2 and Bcl-xL targeted therapy inhibits growth of pancreatic cancer cells. 2006 Gastrointestinal Cancers Symposium; 26–28 Jan 2006; San Francisco, CA, USA.
- Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-Gossypol acts directly on the mitochondria to overcome BcI-2- and Bcl-X(L)-meidated apoptosis resistance. Mol Cancer Ther 2005;4:23-31.
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;91:479-89.
- Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol 2003;66:93-103.
- Li ZM, Jiang WQ, Zhu ZY, Zhu XF, Zhou JM, Liu ZC, et al. Synergistic cytotoxicity of Bcl-xL inhibitor, gossypol and chemotherapeutic agents in non-Hodgkin’s lymphoma cells. Cancer Biol Ther 2008;7:51-60.
- Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. Intermediacy of HO- and p53-dependent pathways. J Biol Chem 2004; 279: 25 535−43.
- Wei S, Liu G, Yang J, Sheng W, Wu H. The experimental research of the induction of apoptosis in human hepatocelluar cancer cell line SMMC-7721 by pharmorubicin. Jiangsu Med J 2002;28:23-5.