Identification of human dopamine D1-like receptor agonist using a cell-based functional assay1
Introduction
The dopamine receptors fall into two families called D1-like and D2-like receptors, based on their structural and pharmacological features[1]. The D1 family includes D1- and D5-receptor subtypes, and the D2 family consists of D2-, D3- and D4-receptor subtypes. Dopamine receptors are mainly expressed in the central nervous system and control motor function, emotional state and endocrine physiology[2,3].
Many central nervous system (CNS), cardiovascular and renal diseases have been shown to be associated with alterations in dopamine receptors. These diseases include Parkinson disease, schizophrenia, migraine, drug dependence, depression and Gilles de la Tourette syndrome[4]. Dopamine is one of the principal neurotransmitters in the basal ganglia, and plays a critical role in motor control and cognitive function through interactions with dopamine receptors. The possible role of dopamine D1-like receptors in brain function, especially in learning and memory, has recently been studied extensively[5–7]. Indeed, D1-like receptors play essential roles in working memory[8] and other forms of cognition activity[9]. The abnormality of these receptors also contribute to Parkinson disease[10,11]. In addition, the D5 receptor subtype is involved in modulating the release of hippocampal acetylcholine, a neurotransmitter implicated in a variety of cognitive processes[12].
Much evidence has been accumulated to indicate that dopamine receptor agonists or antagonists can be developed into therapeutic drugs for the treatment of CNS diseases. It has been reported that D1-like receptor agonists improve learning and memory in different animal models[13,14]. Furthermore, D1-like receptor agonists or antagonists are potential therapeutic drugs for addiction and Parkinson disease[15–18]. Receptor agonists and antagonists also provide essential tools for pharmacological and functional characterization of the receptors.
Several high throughput screening methods to screen agonists and antagonists of G-protein coupled receptors (GPCR) have been developed[19–22]. Recently, we developed a universal functional assay for GPCR[23]. In the present report, we further modified this functional assay for human D1-like agonist screening. A number of natural compounds were identified that can specifically activate both human D1 and D5 receptors. Detailed pharmacological analysis demonstrated that one of the agonists had distinct pharmacological properties for these 2 receptors.
Materials and methods
Plasmid construction Human D1 and D5 receptors were cloned by polymerase chain reaction (PCR). The primers used for the PCR were: D1R5', 5'-GCT
Cell culture, transfection and stable cell line generation CHO cells were maintained in RPMI-1640 medium containing 10% fetal calf serum at 37 °C. Cells were transfected with dopamine D1 and D5 receptors and the reporter construct using Lipofectin (Invitrogen). Stably transfected cells were generated in the presence of 0.8 mg/mL G418.
Natural product extracts Traditional Chinese Medicines (TCM) were purchased from a local pharmacy in Chongqing, China. Fifty grams of each TCM were extracted twice with 500 mL water at 80 °C for 2 h. The extracts were concentrated to 100 mL by evaporation under low pressure at 80 °C. Using this method, we prepared more than 300 samples for human dopamine receptor agonist screening.
Luciferase assay Aliquots of 90 µL cells (3×105 cells/mL) were seeded into each well of 96-well plates and incubated overnight at 37 °C. A natural product sample (10 µL) was added to each well and incubated at 37 °C for 6 h–10 h. Bright-GloTM Lucifease assay reagent (100 µL; Promega) was then added to each well, and the luciferase activity was measured using AnalystTM HT (Molecular Device).
Results
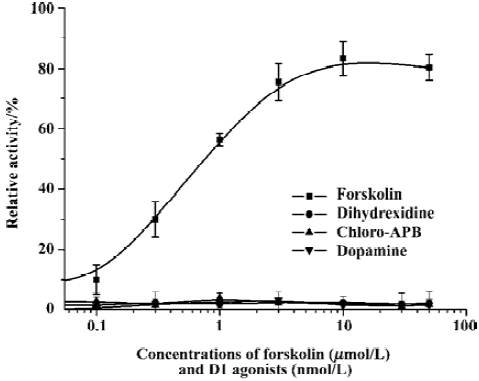
Characterization of the CRE/TA/Luci reporter gene in CHO cells We generated a reporter gene construct for human dopamine D1 and D5 agonist screening. The construct contained 6 copies of CRE and a TATA box linked to the luciferase gene. The reporter gene construct was stably transfected into CHO cells to generate the CRE/TA/Luci/CHO cell line. This cell line was treated with forskolin (an adenyl cyclase activator) at different concentrations, and luciferase activity was measured. Our results showed that forskolin stimulated reporter gene luciferase expression in a dose-dependent manner (Figure 1). This result indicated that increasing intracellular cAMP levels led to the activation of CRE and TATA promoter and suggested that this assay could be used for Gs-coupled receptor agonist screening. To examine whether there was any endogenous dopamine receptor in the CHO cells, we tested several dopamine receptor agonists in the stable reporter gene cell line. Our results demonstrated that none of the dopamine receptor agonists had any effect on the cell line, indicating that no dopamine receptor was expressed in CHO cells.
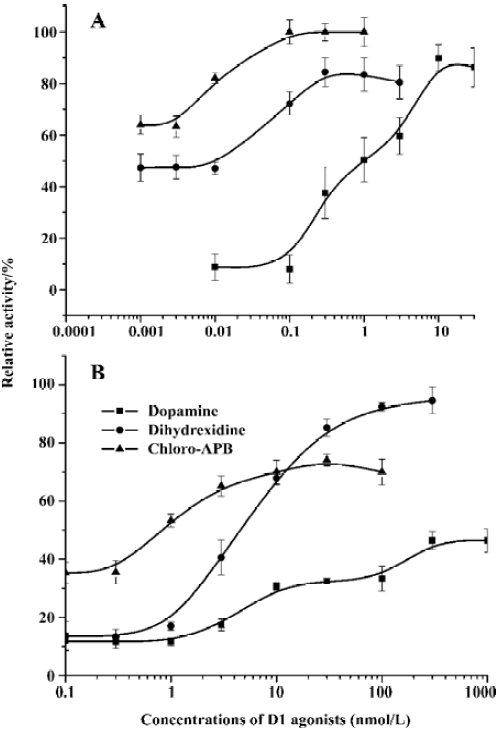
Development of reporter gene assay for D1-like receptor agonist screening Mammalian expression vectors containing human dopamine D1 or D5 receptors were transfected into the reporter gene cell line. Activation of the receptors leads to the elevation of cAMP and activates the cAMP response element, and therefore induces the expression of the reporter gene luciferase. We tested the natural ligand dopamine and two other D1-like receptor agonists, dihydrexidine and chloro-APB, in the stable cell lines expressing both dopamine receptors and the reporter gene. The results are shown in Figure 2. The rank order of potency, dopamine>dihydrexidine>chloro-APB, agreed with the ligand-receptor binding analysis[24,25].
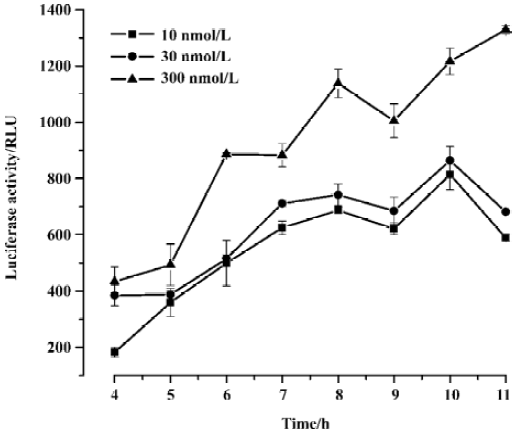
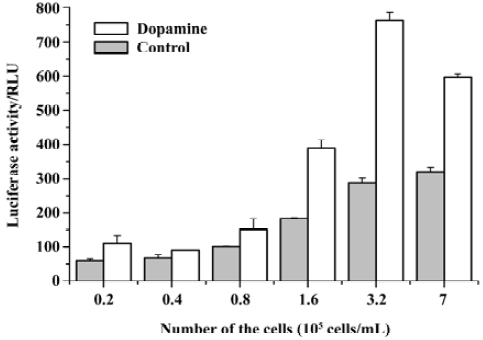
Optimization of the reporter gene assay conditions We carried out a series of experiments to optimize the reporter gene assay for drug screening. First, we examined the incubation time for the D1-like receptor agonist in the assay. Different concentrations of dihydrexidine were used for the experiment. Our results showed that approximately 8 h incubation with the agonist gave the highest response at all concentrations (Figure 3). Therefore, we used an 8-h incubation time for all of our experiments, unless otherwise indicated. Second, because the compounds were in dimethylsulphoxide (Me2SO) solution, we tested the effects of Me2SO at different concentrations in the assay. Me2SO concentrations of 1% or less had no effect on the signal (data not shown). In our compound screen assays, the final concentration of Me2SO was adjusted at 1% or less. Finally, we found that the number of cells in each well may influence the reporter gene assay. Figure 4 shows that although increasing cell number gave a better signal after agonist stimulation, the background was also higher. The best number of cells to use was between 2×105 cells/mL and 4×105 cells/mL. Therefore, we used 3×105 cells/mL in all compound screens using the reporter gene assay. In addition, we calculated the coefficient of variation (CV) for the assay in a standard 96-well plate. The CV values were 6.5% for non-activated cells and 5.8% for dopamine-stimulated cells. The Z’ factor[26] was 0.58. These results suggest that the reporter gene assay system was suitable for dopamine receptor agonist screening.
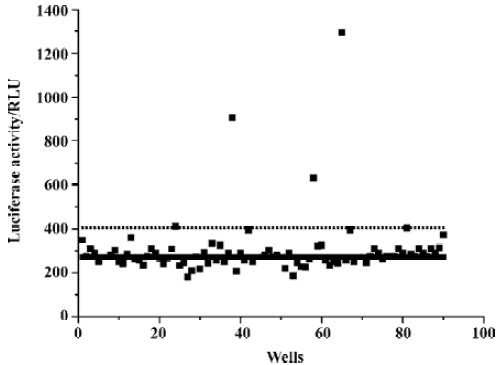
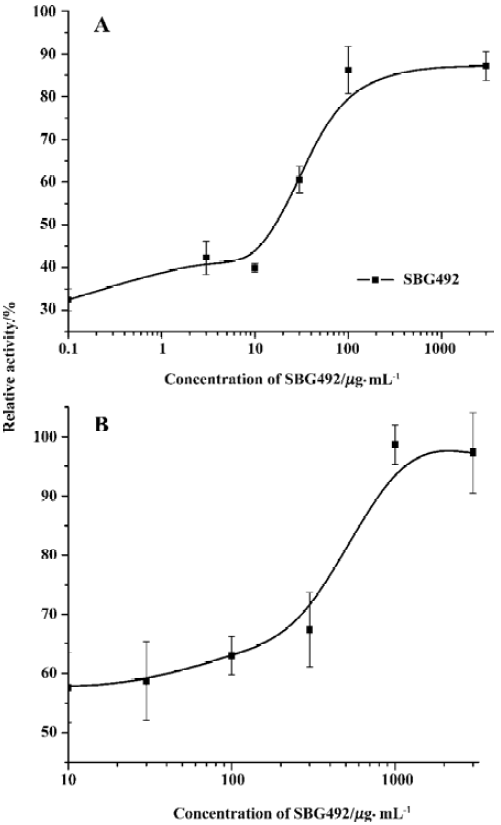
Identification of extracts active for the D1-like receptor More than 300 herb extract samples were used for the D1-like receptor agonist screening. From our previous experiences for other G-protein coupled receptor agonist or antagonist screens, we found that it was necessary to use different concentrations of the raw extracts for the screen. Therefore, in the D1-like receptor agonist screen, the extract samples were screened twice at different concentrations. One was at the original concentration and the other was a 5-fold dilution of the sample. The samples that gave signals larger than the mean value +3SD were selected as agonist candidates (Figure 5). To eliminate the possibility that the agonist candidates activated the reporter gene expression through intracellular pathways other than the D1-like receptor, we tested the samples in a cell line expressing the reporter gene alone, without the D1-like receptor. We found that some of the agonist candidates, such as Chinese ester pillar fungus, could stimulate the reporter gene luciferase expression in the reporter gene cell line (Figure 6). In contrast, sample SBG492 activated the expression of the reporter gene in the D1-like receptor expressing cell line, but had no effect in the reporter gene cell line. This result suggested that SBG492 activated the reporter gene expression through human D1-like receptor (Figure 6). Furthermore, we tested the sample in more than 20 different GPCR using the same reporter gene assay system (data not shown). Our results demonstrated SBG492 could not activate other GPCR, suggesting that the sample contained specific human D1-like receptor agonist. We further analyzed the pharmacological properties of SBG492 for human D1 and D5 receptors. The EC50 values of SBG492 were 342.7 µg/mL for the D1 receptor and 31.7 µg/mL for the D5 receptor (Figure 7).

Discussion
Dopamine receptors are a subclass of the superfamily of GPCR. Within the dopamine receptor family, both D1 and D5 are Gs-coupled receptors. Interaction of the receptors with the natural ligand dopamine or other agonists leads to the activation of adenyl cyclase and increases the intracellular concentration of cAMP. The cAMP second messenger system ultimately activates CRE and induces gene expression. This is the basis of our reporter gene assay, which contains 6×CRE linked to the reporter gene luciferase.
The D1 and D5 dopamine receptors are genetically distinct, sharing over 80% sequence homology within the highly conserved 7 transmembrane-spanning domains, but display only 50% overall homology at the amino acid level[27]. The D1-like dopamine receptors, including D1 and D5, have similar pharmacological properties[28]. Indeed, it is often difficult to pharmacologically distinguish between dopamine D1 and D5 receptors using the same ligands. Therefore, identification of agonists that have distinct pharmacological properties for these 2 receptors should provide a useful tool for their functional studies.
In our previous agonist or antagonist screening for GPCR and other targets, we have successfully isolated active components from crude extracts of herbs[29–31]. Subsequent purification of the active components lead to the identification of a single effective compound. The advantage of using the crude extract is that the samples are easy to prepare and a large amount of herbs can be screened in a short period of time. On the other hand, since the crude extracts contain hundreds of different compounds, some of them may have negative effects on the targets or may even be harmful to cells. Indeed, we found that in some cases, high concentrations of the extracts in our cell-based assay had no effects, while after dilution the samples showed agonist or antagonist activity. Therefore, we used 2 concentrations of each extract for the screening to increase the chances of identifying D1-like receptor agonists. A number of extracts were isolated as potential receptor agonists using the reporter gene assay.
There are several other possible ways in which that the extracts can activate the reporter gene expression other than as human D1-like receptor agonists. For example, the extracts may activate or inhibit other components in the cAMP signal pathway, such as adenyl cyclase, protein kinase A, cAMP-dependent phosphodiesterase or CRE binding protein, and eventually lead to the activation of the reporter gene. It is also possible that the extracts may activate other endogenous GPCR in the cell and lead to the induction of reporter gene expression. To examine these possibilities, we tested the activity of the herb extracts in a reporter gene cell line that did not express human D1-like receptors. We found that SBG492 did not induce the expression of the reporter gene in the cell line, indicating that the extract activated the reporter gene through the receptor. It is interesting to note that SBG492 is approximately 10 times more potent for human D1 receptor than for D5 receptor. Further characterization of the agonist could provide important information for pharmacological and functional studies on human D1-like receptors.
Acknowledgements
We thank Hong GAO, Lu WANG, and Li-ping WANG for their excellent technical assistance.
References
- Braun AR, Laruelle M, Mouradian MM. Interactions between D1 and D2 dopamine receptor family agonists and antagonists: the effects of chronic exposure on behavior and receptor binding in rats and their clinical implications. J Neural Transm 1997;104:341-62.
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev 2000;24:125-32.
- Meyer ME, Cottrell GA, Van Hartesveldt C, Potter TJ. Effects of dopamine D1 antagonists SCH23390 and SK&F83566 on locomotor activities in rats. Pharmacol Biochem Behav 1993;44:429-32.
- Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors-physiological understanding to therapeutic intervention potential. Pharmacol Ther 1999;84:133-56.
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 1994;79:729-42.
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O’Dowd BF, George SR. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol 1999;383:95-106.
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, et al. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci 2003;23:8506-12.
- Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: immunocytochemical and cognitive neuropharmacological analyses. Synapse 2003;47:101-8.
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 1995;376:572-5.
- Hurley MJ, Mash DC, Jenner P. Dopamine D(1) receptor expression in human basal ganglia and changes in Parkinson’s disease. Brain Res Mol Brain Res 2001;87:271-9.
- Mailman R, Huang X, Nichols DE. Parkinson’s disease and D1 dopamine receptors. Curr Opin Invest Drugs 2001;2:1582-91.
- Hersi AI, Kitaichi K, Srivastava LK, Gaudreau P, Quirion R. Dopamine D-5 receptor modulates hippocampal acetylcholine release. Brain Res Mol Brain Res 2000;76:336-40.
- Castellano C, Cestari V, Cabib S, Puglisi-Allegra S. Post-training dopamine receptor agonists and antagonists affect memory storage in mice irrespective of their selectivity for D1 or D2 receptors. Behav Neural Biol 1991;56:283-91.
- Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J Neurosci 2004;24:1446-50.
- Linazasoro G. Conversion from dopamine agonists to prami-pexole. An open-label trial in 227 patients with advanced Parkinson’s disease. J Neurol 2004;251:335-9.
- Jenner P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson’s disease. Curr Opin Neurol 2003;16 Suppl 1:3-7.
- Ruggieri S, Stocchi F, Baronti F, Viselli F, Horowski R, Lucarelli C, et al. Antagonist effect of terguride in Parkinson’s disease. Clin Neuropharmacol 1991;14:450-6.
- Meyer ME, Van Hartesveldt C, Potter TJ. Locomotor activity following intra-accumbens microinjections of dopamine D1 agonist SK&F 38393 in rats. Synapse 1993;13:310-4.
- Kariv II, Stevens ME, Behrens DL, Oldenburg KR. High throughput quantitation of camp production mediated by activation of seven transmembrane domain receptors. J Biomol Screen 1999;4:27-32.
- Fitzgerald LR, Mannan IJ, Dytko GM, Wu HL, Nambi P. Measurement of responses from Gi-, Gs-, or Gq-coupled receptors by a multiple response element/cAMP response element-directed reporter assay. Anal Biochem 1999;275:54-61.
- George SE, Bungay PJ, Naylor LH. Functional analysis of the D2L dopamine receptor expressed in a cAMP-responsive luciferase reporter cell line. Biochem Pharmacol 1998;56:25-30.
- Hertzberg RP, Pope AJ. High-throughput screening: new technology for the 21st century. Curr Opin Chem Biol 2000;4:445-51.
- Jiang C, Chen G, Zeng X, Ouyang K, Hu Y. Generation of a bioactive neuropeptide in a cell-free system. Anal Biochem 2003;316:34-40.
- Gilmore JH, Watts VJ, Lawler CP, Noll EP, Nichols DE, Mailman RB. “Full” dopamine D1 agonists in human caudate: biochemical properties and therapeutic implications. Neuropharmacology 1995;34:481-8.
- Grandy DK, Zhang YA, Bouvier C, Zhou QY, Johnson RA, Allen L, et al. Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proc Natl Acad Sci USA 1991;88:9175-9.
- Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999;4:67-73.
- Sidhu A. Coupling of D1 and D5 dopamine receptors to multiple G proteins: Implications for understanding the diversity in receptor-G protein coupling. Mol Neurobiol 1998;16:125-34.
- Seeman P, van Tol HH. Dopamine receptor pharmacology. Curr Opin Neurol Neurosurg 1993;6:602-8.
- Xu ZL, Gao H, Ou-Yang KQ, Cai SX, Hu YH. Establishment of a cell-based assay to screen regulators for Klotho gene promoter. Acta Pharmacol Sin 2004;25:1165-70.
- Zheng XX, Ou-Yang KQ, Gao H, Xu ZL, Hu YH, Cai SX. Study on high throughput screening method of identifying agonist for neuromedin U2 receptor. Chin Pharm J 2004;9:185-8. Chinese..
- Gao H, Ou-Yang KQ, Zheng XX, Xu ZL, Hu YH, Cai SX. High throughput screening method of identifying agonist for muscarinic receptor. Chin Pharmacol Bull 2003;19:776-9. Chinese..
