A robust homogeneous binding assay for α4β2 nicotinic acetylcholine receptor1
Introduction
Nicotinic acetylcholine receptors (nAChR) are excitatory ligand-gated ion channels mainly distributed in the central and peripheral nervous systems, neuromuscular junctions and adrenal glands[1]. The nAChR channel complex is composed of 5 protein subunits, which form a pore that is permeable to Na+, K+, and Ca2+. To date, α, β, γ, δ, and ε subunits have been cloned, with 9 different α and 4 varieties of β subunits identified[2]. The α4β2 is the predominant nAChR subtype in the mammalian brain and has a high affinity for nicotine. nAChR consisting of α4 and β2 subunits modulates neurotransmitter release and plays a direct role in nicotine addiction. This group of nAChR was implicated in the pathological pathways of Alzheimer disease, Parkinson disease, schizophrenia, epilepsy and pain[3].
The anti-nociceptive effects of (–)-nicotine have been known for over 60 years. This non-selective nAChR agonist could not be applied as an analgesic agent due to serious adverse events. Although (–)-epibatidine is a highly selective α4β2 nAChR agonist that displays a more potent analgesic activity than morphine in nociceptive tests[4], toxicities in the cardiovascular and central nervous systems rendered it unsuitable for therapeutic use[5]. Therefore, a primary goal in the development of novel analgesic agents based on α4β2 nAChR is to discover subtype-specific agonists with less liability.
An accurate and robust receptor-binding assay is highly desirable to screen a large collection of chemical entities. Associated techniques are generally based on absorbance, fluorescence and radiometric assays. Scintillation proximity assay (SPA), because of its simplicity and high-throughput nature, has broad applications in measuring receptor-ligand interactions and enzyme reactions. Conventional filter binding assays involve 1 or more separation steps and, thus, are laborious, non-homogeneous and not suitable for automation. SPA technology[6], on the other hand, provides a homogeneous screening approach that does not require post-reaction liquid handling steps and is well suited to automation and high-throughput screening (HTS). Briefly, the receptor is anchored to a scintillant-impregnated microbead. When an isotope (eg, [3H]) is brought very close to the microbead by binding to its surface, it activates the scintillant leading to light emission. Because the emitted β particles or augur electrons can only travel short distances in the bulk solution, the microbead preferentially captures electrons from the bound radiolabeled ligand. Therefore, the amount of light emitted from the scintillant in the microbead is directly proportional to the amount of bound radiolabeled ligand. In the present paper, we describe a simple SPA method to assess specific binding properties of various known ligands to α4β2 nAChR. It was further validated and optimized in the context of HTS for a library containing 32 000 synthetic compounds. More than a dozen compounds with novel chemical structures were found to have relatively high binding affinity to α4β2 nAChR and 8 of them showed significant antagonist activities in a functional assay.
Materials and methods
Reagents Potassium chloride, sodium phosphate monobasic anhydrous, and magnesium chloride haxahydrate were purchased from Shanghai Chemical Co (Shanghai, China). Aprotinin and leupeptin were purchased from Merck KGaA (Darmstadt, Germany). RJR 2403 and epibatidine were purchased from Sigma-Aldrich (St Louis, MO, USA). [3H]Cytisine (37 Ci/mmol) was obtained from Amersham Biosciences UK (Buckinghamshire, UK). FlashBlue™ GPCR beads, Isoplate™ and Filtermat B made of glass fiber were bought from PerkinElmer Life and Analytical Sciences (Boston, MA, USA).
Cell culture and membrane preparation The full-length cDNA of the α4 (GenBank Accession N
Filter binding assay Various amounts of the above membrane receptor preparation, 3 nmol/L [3H]cytisine and different concentrations of known ligands to α4β2 nAChR were added to the binding buffer (50 mmol/L Tris HCl, 120 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 2.5 mmol/L CaCl2, pH 7.4) to give a final volume of 0.1 mL. After incubation at 4 °C for 8 h, the membrane fraction was harvested on the Filtermat B presoaked with a solution consisting of the washing buffer (50 mmol/L Tris HCl, pH 7.4) and 0.5% (v/v) polyethyleneimine. The Filtermat B was washed 3 times with the washing buffer and dried at 55 °C for 45 min before counting on a Microbeta scintillation counter (PerkinElmer). Non-specific binding activity was determined in the presence of 2 µmol/L epibatadine.
SPA binding assay Various amounts of the above membrane receptor preparation, 3 nmol/L [3H]cytisine, FlashBlue™ GPCR beads (62.5 µg/well) and different concentrations of known ligands to α4β2 nAChR were added to the binding buffer to give a final volume of 0.1 mL. The plates were incubated at 4 °C for 12 h and centrifuged for 3 min at 2500×g before counting on the Microbeta scintillation counter.
Calcium mobilization assay The above cells expressing α4β2 nAChR were detached and loaded with 5 µmol/L Fluo-4 (Molecular Probes, Eugene, OR, USA) in culture medium supplemented with 2.5 mmol/L probenecid (Sigma) for 45 min. They were then washed twice with solB buffer containing: 10 mmol/L HEPES, 5 mmol/L NaCl, 5 mmol/L KCl, 2 mmol/L CaCl2, 140 mmol/L N-methyl-D-glucamine and 10 mmol/L glucose (pH 7.4). The cells were resuspended in solB buffer, plated onto 96-well plates at a density of 60 000 cells in 80 µL per well, and reattached by centrifugation. Effects of the hit compounds on calcium influx were analyzed by FlexStation™ (Molecular Devices, Sunnyvale, CA, USA) with excitation wavelength 485 nm and emission wavelength 525 nm. An α4β2 nAChR agonist, ABT-594[8], was used as a positive control.
HTS campaign The compound library used for screening consisted of 32 000 pure synthetic compounds. A 10-compound pool per well mix was applied to the primary screening, with an average concentration of 7 µmol/L for each compound dissolved in 100% dimethylsulphoxide (Me2SO) solution. This matrix system maximizes the advantage of HTS and allows duplicate screening of each compound[9]. In each 96-well IsoplateTM, 16 wells were used as positive controls (epibitadine) and samples showing greater than 60% inhibition were considered as “hits”.
Data analysis Data were analyzed using GraphPad Prism software (GraphPad, San Diego, CA, USA). Non-linear regression analyses were performed to generate dose-response curves. Ki values were calculated from IC50 using the equation of Cheng and Prusoff[10].
Results
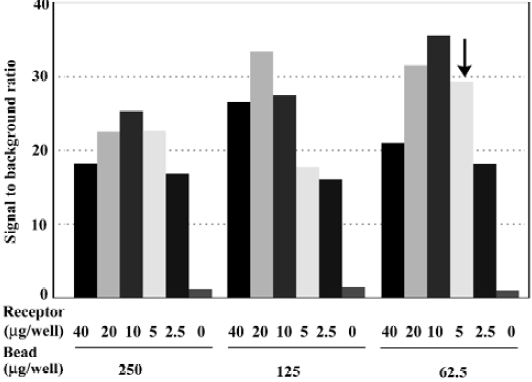
Assay optimization For HTS purposes, 96-well microtiter plates were used and reaction volume was adjusted to 100 µL/well. At a concentration equivalent to the previously reported Kd value (~0.5 nmol/L)[11], more than 25% of [3H]cytisine added was bound to the receptor (data not shown). To avoid excessive ligand depletion, the concentration was increased to 3 nmol/L, in which the bound form accounted for less than 10%. The best signal-to-background (S/B) ratio was observed in a matrix experiment when 62.5 µg/well microbeads and 10 µg/well membrane preparation were used. However, 5 µg/well was selected for the HTS campaign to conserve the membrane preparation. Under these assay conditions, a sound S/B ratio (~30) was achieved (Figure 1). Me2SO, at concentrations below 3%, did not affect the assay performance (data not shown).
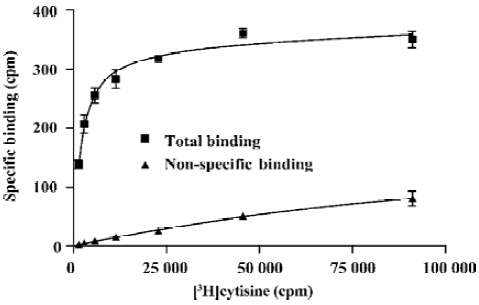
Binding saturation A serial titration of [3H]cytisine was prepared to study binding characteristics of the SPA assay (Figure 2). The Bmax and Kd values calculated from the SPA and filter binding assays were strikingly similar: 2.26 pmol/mg vs 2.31 pmol/mg and 0.65 nmol/L vs 0.70 nmol/L, respectively.
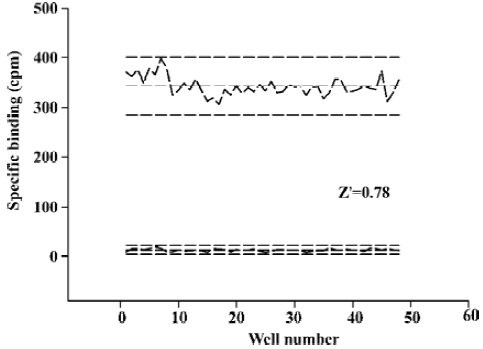
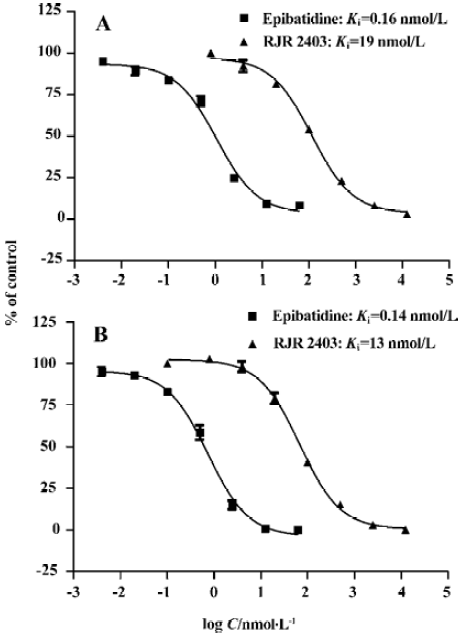
Assay performance As shown in Figure 3, the average Z’ value for the SPA binding assay was 0.78 with a S/B ratio of 29, indicating that the system was adequately optimized for HTS. Assay stability was evaluated by incubating the plates overnight at 4 °C and both the Z’ factor and the S/B ratio remained unchanged. Two known α4β2 nAChR ligands, namely, epibatidine and RJR2403, were used to compare the 2 assay methods and the binding affinities measured were within the same range (Figure 4).
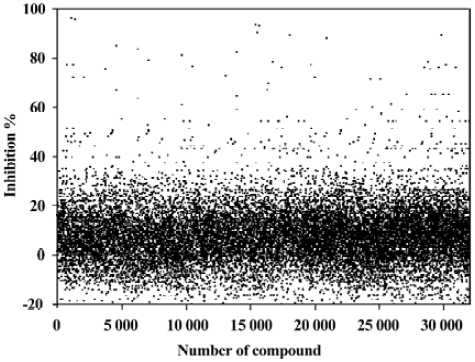
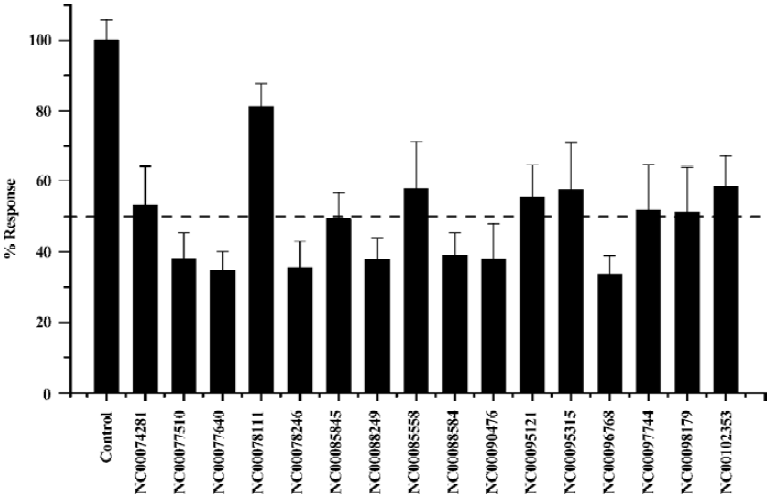
HTS campaign Of the 32 000 samples initially screened, 54 hits (0.17%) showing greater than 60% competitive inhibition on [3H]cytisine binding to α4β2 nAChR were discovered (Figure 5). Secondary (single compound per well) screening confirmed that 17 of the above hits displayed consistent inhibitory effects with Ki values below 2 µmol/L. These active compounds are of different chemical structures including thiophene, piperidine, azole and other types of heterocyclic derivatives. The HTS campaign was high quality in nature with a Z’ factor and S/B ratio equaling 0.73 and 29, respectively. The bioactivities of the confirmed hits were further evaluated with a functional assay. Eight compounds displayed antagonistic effects with >50% inhibition at 6.7 mg/L on ABT-594-induced calcium mobilization while none showed any agonist activity (Figure 6).
Discussion
Taking advantage of our prior experience in the development of a SPA-based nuclear receptor binding HTS method[12], efforts were made to expand the knowledge to membrane receptors such as α4β2 nAChR described in this paper. Although similar SPA approaches have been employed to study ligand-binding characteristics of a variety of G protein-coupled receptors[6,13–15], its application in α4β2 nAChR is novel and may be expandable to other ligand-gated ion channels.
When developing a SPA-based receptor-binding assay, many factors have to be considered in order to maximize the S/B ratio. The optimization procedure may include scintillant-impregnated microbead selection, assay buffer and volume determination, or protein/bead ratio verification. While the assay buffer could be readily transferred from a conventional filter binding assay, the amount of receptor protein and its relative ratio to the quantity of beads used are critical to assay performance. An adequate amount of beads is required to saturate the protein in order to achieve a maximal binding signal. However, excessive beads will lead to undesired exposure of bead surface to non-specific ligand binding. Therefore, the amount of beads applied should be kept to a minimum where protein saturation is still achievable. On the other hand, stable signal is highly dependent on protein stability and its coating ability on the beads. In the assay system reported here, no signal shift was observed for more than 48 h, indicating a very stable interaction among various reagents.
It was learned from other membrane receptor screening settings that assay sensitivity can be improved by choosing a radiolabeled ligand concentration at or below its Kd to permit effective competition by an unlabeled ligand[14]. In the present study, we selected a relatively higher concentration of [3H]cytisine (ie, 3 nmol/L) as opposed to the Kd value (0.6 nmol/L) of unlabeled ligand. This arrangement allowed us to accurately measure Ki values of the 2 reference compounds as well as to confirm hits identified from the HTS campaign, while keeping [3H]cytisine bound to the receptor to a minimum (<10%). Conceivably, such a practice would compromise assay sensitivity thereby reducing the hit rate. Considering our initial intention of finding hits with Ki values below 2 µmol/L from primary screening, this purpose was well served as evidenced by a confirmed hit rate of 0.05% and the identification of 1 highly active compound (23 nmol/L; data not shown). In addition, the bioactivities of the hits discovered by the SPA method were confirmed with a cell-based functional assay where 8 compounds were found to demonstrate significant antagonistic effects on ABT-594-induced calcium mobilization.
In comparison with conventional filter binding techni-ques, the SPA method omits steps such as pre-coating, pre-incubation, separation and excessive washing, and thus, simplifies the assay protocol, mitigating labor intensity and reducing systemic error. It is also readily adaptable to HTS and automation, as demonstrated in this study. When deduced to practice, both approaches yielded similar Ki values for 2 known α4β2 nAChR ligands (epibatidine and RJR2403), which are not only consistent with those reported elsewhere[16] but also exhibited the same affinity rank order and pKi features (in agreement within 1/2 log unit). In addition, the cost and amount of wastage are significantly less if SPA technology is employed. This advantage could be further explored by utilizing high-density plate formats (eg, 384-well plate) where filtering assays clearly show their limitations.
The Z’ factor is a useful tool for evaluating bioassay qualities[17]. In general, a Z’ value above 0.5 suggests that an assay is robust enough for HTS. The SPA system described herein consistently displayed a Z’ value between 0.73 and 0.78. This, and in conjunction with other parameters such as S/B ratio, Bmax and Kd values, indicate that the assay is high quality in nature.
In summary, a simple and SPA-based HTS binding assay was developed and validated for identification of compounds with specificity and functionality for α4β2 nAChR. Receptor coating onto the beads and ligand binding are achieved in 1 mixing step and the procedure is homogeneous. Its application may be expanded to other membrane receptors and ion channels.
Acknowledgements
We are indebted to Mr Bin WU and Ms Juan MA for their technical assistance, and to Dr DE MAIS for his valuable comments and critical review of this manuscript.
References
- Lukas RJ, Changeux JP, Novere N, Albuquerque EX, Balfour DJ, Berg DK, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 1999;51:397-401.
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, et al. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 1997;280:1117-36.
- Cordero-Erausquin M, Marubio LM, Klink R, Changeux JP. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol Sci 2000;21:211-7.
- Kesingland AC, Gentry CT, Panesar MS, Bowes MA, Vernier JM, Cube R, et al. Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain 2000;86:113-8.
- Boyce S, Webb JK, Shepheard SL, Russell MG, Hill RG, Rupniak NM. Analgesic and toxic effects of ABT-594 resemble epibatidine and nicotine in rats. Pain 2000;85:443-50.
- Carpenter JW, Laethem C, Hubbard FR, Eckols TK, Baez M, McClure D, et al. Configuring radioligand receptor binding assays for HTS using scintillation proximity assay technology. Methods Mol Biol 2002;190:31-49.
- Chavez-Noriega LE, Gillespie A, Stauderman KA, Crona JH, Claeps BO, Elliott KJ, et al. Characterization of the recombinant human neuronal nicotinic acetylcholine receptors α3β2 and α4β2 stably expressed in HEK293 cells. Neuropharmaco-logy 2000;39:2543-60.
- Donnelly-Roberts DL, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, et al. ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: a novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: In vitro characterization. J Pharmacol Exp Ther 1998;285:777-86.
- Qian J, Voorbach MJ, Huth JR, Coen ML, Zhang HC, Ng SC, et al. Discovery of novel inhibitors of Bcl-xL using multiple high-throughput screening platforms. Anal Biochem 2004;328:131-8.
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099-108.
- Khan IM, Yaksh TL, Taylor P. Ligand specificity of nicotinic acetylcholine receptors in rat spinal cord: studies with nicotine and cytisine. J Pharmacol Exp Ther 1994;270:159-66.
- Wu B, Gao J, Wang M-W. Development of a complex scintillation proximity assay for high-throughput screening of PPARγ modulators. Acta Pharmacol Sin 2005;26:339-44.
- Crane K, Shih DT. Development of a homogeneous binding assay for histamine receptors. Anal Biochem 2004;335:42-9.
- Gobel J, Saussy DL, Goetz AS. Development of scintillation-proximity assays for alpha adrenoceptors. J Pharmacol Toxicol Methods 1999;42:237-44.
- Rodgers G, Hubert C, McKinzie J, Suter T, Statnick M, Emmerson P, et al. Development of displacement binding and GTPγS scintillation proximity assays for the identification of antagonists of the micro-opioid receptor. Assay Drug Dev Technol 2003;1:627-36.
- Xiao Y, Baydyuk M, Wang HP, Davis HE, Kellar KJ. Pharmacology of the agonist binding sites of rat neuronal nicotinic receptor subtypes expressed in HEK 293 cells. Bioorg Med Chem Lett 2004;14:1845-8.
- Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999;4:67-73.
