Estrogen plays a critical role in AAV2-mediated gene transfer in ovarian cancer1
Introduction
Ovarian cancer due to gynecological malignancy is the leading cause of death among women. Despite advances in surgery and chemotherapy, the long-term survival for advanced-stage ovarian cancer rarely exceeds 15%–30%, and approximately 90% of patients have the disease contained with peritoneal cavity metastasis at initial diagnosis. Gene therapy is the most effective alternative therapeutic approach and has been widely investigated. Virus-deprived delivery systems have been studied for therapeutic gene transfer and transduction, such as p53, interleukin-12, and herpes simplex virus thymidine kinase (HSV-tk). Tissue-specific promoters that target gene expression in ovarian cancer cells, and ovarian cancer-directed, modified adenoviral vectors have also been widely investigated and reported[1–7].
The adeno-associated virus (AAV) is a most effective alternative vector for gene therapy. It has been reported that recombinant AAV (rAAV)2, with the insertion of the target peptide into the capsid, could retarget tumor cells[8–11]. In our laboratory, we successfully invented the luteinizing hormone or arginine-glycine-aspartic acid sequence (RGD) peptide-modified rAAV2, both of which exert enhanced selectivity in gene delivery to ovarian cancer cell lines[8,9]. Heparin sulfate proteoglycan, fibroblast growth factor, and integrins have been found to be involved in the attachment, entry and uncoating of rAAV2[12,13]. For its general expression on most transformed cells, integrins are potential targets for cancer therapy, and studies have confirmed this point using adenoviral vectors[5,7,9]. rAAV2 is not as efficient as adenoviral vectors in delivering therapeutic genes into tumor cells[14]. Its low transduction on different ovarian cancer cells is mostly because of the low expression of the AAV receptor heparan sulfate proteoglycan (HSPG) and coreceptor, and its insufficient uncoating process[9,15]. There is a wide variation of HSPG and integrin expressions among ovarian cancer cells in vivo and in vitro. Furthermore, a low HSPG expression definitely reduces rAAV2-mediated gene transduction at the first step. Our previous work confirmed that RGD-introduced alternative interactions can overcome low HSPG expression on ovarian cancer[15]. As reported by many groups, there is lower gene transduction mediated by rAAV2 in female mice than in male ones in different tissues and organs[16,17]. However, there is no detailed study showing the cause of this gene transduction difference between male and female mice. Therefore, there is a need to determine whether sex hormones and their metabolites affect gene transfer and expression by rAAV2 in vivo. Furthermore, in in vivo microenvironments, ovarian cancer cells respond differently to female hormones, such as estrogen and progesterone, which might be associated with alternative therapeutic effects from either chemotherapy or biotherapy.
As heparin sulfate is the primary receptor for AAV2 and critical limit for AAV2 gene transduction in vitro and in vivo, HSPG-mediated viral attachments and internalization should be the main focus of gene delivery and gene transduction. The RGD peptide has been considered a targeting peptide for different tumors, including ovarian cancer[9,15,19–22]. The inserted RGD peptide exerted specific interaction with integrins on the cell surface. We reported that a novel rAAV2 vector, A520/N584RGD-rAAV, featured with depleted natural tropism and alternative integrin interactivity, mediated efficient gene transduction in low-expressing HSPG cells[20]. However, ovarian cancer is closely related with sex hormones in vivo, and estrogen has been reported to stimulate the proliferation of ovarian cancer cells. Therefore, it has been suggested that anti-estrogen therapy should be used in combination with ovarian cancer chemotherapy. Estrogen has also been reported to be the cause of chemoresistance in ovarian cancer. However, there are no studies reported as to whether female hormones, such as estrogen, would affect integrin expression on ovarian cancer cells, which has been shown to be upregulated in transformed cells and more broadly generated on ovarian cancer cells than HSPG.
Therefore, we sought to investigate the influence of estradiol, an estrogen metabolite, on rAAV2 gene transduction in ovarian cancer cells in vitro. This study provides information about the future application of AAV2 vectors in ovarian cancer gene therapy. In the present study, we compared gene expression profiles, such as HSPG and integrins β3 and β5 in different ovarian cancer cell lines, SKOV-3ip, OV-3, and OVCAR3 in the presence/absence of estradiol. We also investigated AAV2 gene transduction and cell proliferation with/without estradiol. We found that SKOV-3ip and OV-3 exerted a significant response towards estradiol, such as increased integrin β5 expression and cell proliferation. However, OVCAR-3 did not show any significant response to estradiol in gene expression or cell proliferation. Our data show that A520/N584RGD-rAAV2 transduced ovarian cancer cells more efficiently than rAAV2, and achieved higher gene transduction in the presence of estradiol due to the upregulation of integrin β5.
Materials and methods
Cell lines and recombinant AAV vectors The human ovarian cancer cell lines OV-3, SKOV-3ip, and OVCAR-3 were purchased from ATCC (Manassas, VA, USA). OVCAR-3 and SKOV-3ip were estrogen receptor α and β positive. The transformed human embryo kidney (HEK)-293 and human cervical carcinoma (HeLa) cell lines were maintained in our laboratory. The HEK-293 and HeLa cell lines were maintained in 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM). OV-3 and SKOV-3ip were maintained in 10% FBS in DMEM/F12. OVCAR-3 was maintained in 20% FBS in RPMI-1640 containing 2 mmol/L L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, and 0.01 mg/mL bovine insulin. All of the cell culture media and supplements were obtained from Gibco Life Technologies (Gaithersburg, MD, USA) and contained penicillin (100 U/mL) and streptomycin (100 U/mL). The cell lines were cultured in either 96-, 24-, or 6-well tissue culture plates, or in flasks at 37 ºC under 5% CO2 in a humidified incubator. A low passage number (20–40) HEK-293, and HeLa cells were preferred in the all of the experiments.
rAAV vectors containing enhanced green fluorescent gene (eGFP) or β-lactase Z (β-LacZ) under the control of the human cytomegalovirus (CMV) immediate-early (IE) promoter/enhancer region were packaged into either wild-type or A520/N584RGD-4C capsids, as described previously[9,20]. The A520/N584RGD-4C vector contains the RGD peptide inserted in the viral capsid protein enabling this vector to infect integrin-expressing cells independent of HSPG. Vectors were produced from adenovirus-infected packaging cell lines, as described previously[23]. Briefly, the cells were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS), and recombinant viruses were released by freezing and thawing 3 times. The crude lysate was clarified by centrifugation at 500×g for 10 min and treated with benzonase at 250 U/mL at 37 °C for 30 min. Viruses were further purified by iodixanol step gradient and heparin sulfate chromatography, and stored at –20 °C in PBS containing 20% glycerol. Viral particle titers were determined by ELISA, DNA dot blot, and real-time PCR, as previously described[8]. All of the ovarian cancer cell lines were grown in the steroid hormone-free FBS when subjected to estradiol biofunctional experiments, such as cell proliferation, HSPG expression, integrin expression, gene transduction, and cell cytotoxicity.
Antibodies and other agents The anti-HSPG monoclonal antibody (mAb) HepSS-1 (Seikagaku, Tokyo, Japan), anti-ανβ3 integrin mAb LM609, and anti-ανβ5 integrin mAb P1F6 were purchased from Chemicon International (Temecula, CA, USA). All of the polyclonal antibodies were from Chemicon and Vector Laboratories (Burlingame, CA, USA). The CellTiter 96(R) AQueous Non-Radioactive Cell Proliferation Assay (MTS) proliferation kit was from Promega (Madison, WI, USA). The anti-estrogen receptor α and β mAb were purchased from Jinmei (Shanghai, China). 17β-Estradiol was from Sigma (St Louis, MO, USA). Charcoal/dextram steroid hormone-free FBS was purchased from Hyclone (Logan, UT, USA).
Flow cytometric analysis of HSPG and integrin expression The flow cytometric analysis of the expressions of HSPG and integrins ανβ3 and ανβ5 was carried out as previously described[5,7,9]. Briefly, the cells growing in the T75 flask were released from the flask by the addition of EDTA and trypsin, and resuspended in SM buffer (HEPES-buffered saline and 1% bovine serum albumin) at 2×106 cell/mL. A total of 100 000 cells were incubated with LM609–fluorescein-isothiocyanate (FITC) and PIF6–FITC at a dilution of 1:200 for 2 h at 4 °C, respectively; the total volume was 500 µL. The cells were then washed with SM buffer 5 times under dark conditions. Ten thousand cells were analyzed by flow cytometry at the Children’s (FACS) Core Facility (Columbus, OH, USA). For the HSPG expression test, the cells were resuspended in SM buffer at 4×106 cell/mL. A total of 200 000 cells were incubated with 500 µL anti-HSPG mAb [immunoglobulin M (IgM)] at dilution of 1:200 at 4 °C for 2 h. The cells were then washed with SM buffer 5 times and incubated with secondary FITC-labeled goat antimouse IgM serum (1:800) for 1 h at 4 °C. After the cells were washed with SM buffer 3 times, 10 000 cells were analyzed by flow cytometry at the Children’s FACS Core Facility. For the further analysis of the effect of 17β-estradiol on HSPG and the expressions of integrins ανβ3 and ανβ5, the cells were first cultured in their own proper medium containing 10% steroid hormone-free bovine serum. After washing 3 time with PBS, the cells were divided equally and cultured for another 24 h. The medium was then replaced with fresh medium, 10% steroid hormone-free FBS containing 17β-estradiol at concentrations of 1×10–12, 1×10–10, and 1×10–8 mol/L. The control group cells were cultured in the same medium, except that estradiol was replaced with the same amount of ethanol (less than 0.01%). Seventy two hours later, the flow cytometric analysis of HSPG and integrins ανβ3 and ανβ5 expressions were performed, as described earlier. Data were presented as the relative expression compared to the non-steroid treatment group.
Analysis of AAV-mediated gene transduction For analysis of the GFP and β-galactosidase (β-gal) gene expression, cells amounting to 1×105 in 0.5 mL complete medium were seeded onto the 24-well plate after trypsination with 0.25% trypsin and EDTA. The plate was then incubated at 37 °C under 5% CO2 in a humidified incubator. After overnight incubation, the medium was switched to 2% FBS DMEM containing either rAAV2–CMV–β-gal or rAAV–CMV–eGFP, respectively, at a multiplicity of infection (MOI) of 500. After 4 h of incubation, free rAAV2 viruses were removed with 10% complete medium. Gene transduction efficiency was evaluated after another 48 h of incubation. The cells were harvested and 10 000 cells were analyzed for GFP gene expression by flow cytometry. For the β-gal expression, the cells were fixed and stained as described previously[8]. The same protocol was followed for the estimation of A520/N584RGD–rAAV2–eGFP. Data were presented as the percentage of transduced cells in the cell population infected at the indicated particle multiplicity. For the investigation of the influence of estradiol on AAV gene transduction, the cells were first cultured in their own proper medium containing 10% steroid hormone-free bovine serum. After washing 3 times with PBS, the cells were seeded onto the 6-well plate in triplicate wells at a density of 1000 per well and cultured for another 24 h. The medium was then replaced with fresh medium containing 17β-estradiol at concentrations of 1×10–12, 1×10–10, and 1×10–8 mol/L. Seventy two hours later, the cells were infected with rAAV2–CMV–eGFP and A520/N584RGD–rAAV2–CMV–eGFP at a MOI of 500 using the earlier-described procedure
Cell growth assay The process followed was that of Young and Samulski with some adjustments[7]. To estimate the effect of 17β-estradiol on the growth rate of the ovarian cancer lines, SKOV-3ip and OVCAR-3 were first grown for 3 d in their own proper medium containing 10% of steroid hormone-free FBS. After washing 3 times, the cells were seeded onto flat-bottomed 96-well plates in hormone-free FBS complete medium. The number of each cell varied depending on cell growth and cell size. Twenty four hours later, the medium was replaced by fresh hormone-free medium containing 17β-estradiol at the following concentrations: 1×10–12, 1×10–10, and 1×10–8 mol/L (initially dissolved in ethanol). The final concentration of ethanol was less than 0.01%. This concentration has been found to show no effect on the proliferation of the cells. The control group of cells was grown in fresh hormone-free complete medium containing an equal amount of ethanol. Four days later, 100 µL of proper medium containing 20 µL MTS was added to each well to evaluate the cell counts. After 1–4 h of incubation, absorbance at 492 nm was measured by an ELISA reader. To keep the measured cell numbers in the linear range of MTS assay, the initial seeded cell number was adjusted to the individual proliferation rate of each cell line. Data in all experiments represent the mean of 3–5 samples for each determination.
Ganciclovir sensitivity assay for ovarian cancer cells transduced with rAAV2 carrying herpes simplex virus thymidine kinase (HSV-tk) Both ovarian cancer cells were grown in hormone-free complete medium for at least 3 d before plating onto 96-well plates for the ganciclovir (GCV) sensitivity assay. The SKOV-3ip and OVCAR-3 cells were seeded onto 96-well plates at a density of 2.5×103 and 1.5×103 cells per well, respectively, and exposed to either the rAAV2–HSV-tk vector or A520/N584RGD–rAAV–HSV-tk at a multiplicity of 1000 DNase-resistant particle (DRP)/cell 24 h after plating. After 24 h of incubation, the medium was replaced with fresh medium containing 20 µg/mL of GCV. The cells were then cultured at 37 °C for another 7 d, and the number of viable cells was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For the analysis of 17β-estradiol, the cells were incubated with estradiol 72 h before being incubated with rAAV2. The cytotoxic results are expressed as the number of cells in the wells containing the drug subtracted from the number of cells in the corresponding drug-free controls (bars, SD). The cytotoxic results for the cells transduced with the AAV2–EGFP control vector and treated with GCV were less than 2% for all of the cell lines assayed.
Statistical analysis The MTT assay for cell viability and the cell proliferation test were repeated 4 times; all the results were parallel. The optical density (OD) values in the MTT assay were presented by one experiment. The HSPG and integrin expression analysis by flow cytometry was repeated twice. Data were considered statistically significant when P<0.05.
Results
rAAV2 variably transduces ovarian cancer cells To evaluate whether rAAV2 could be used as a potential gene delivery tool for ovarian cancer gene therapy, ovarian cancer cells were infected with rAAV2–LacZ vectors at a MOI of 100. Consistent with previous studies, OVCAR-3 was permissive to rAAV2, and SKOV-3ip was resistant, as tested by cytohistochemical staining (Figure 1A, 1D). The majority of the OVCAR-3 cells were stained blue, while only a minority of SKOV-3ip cells were stained blue. There were more blue-stained OVCAR-3 cells than SKOV-3ip cells with the same MOI infection, indicating that better gene transfer efficiency was achieved in OVCAR-3 cells than SKOV-3ip (Figure 1). However, even at a 10-fold higher MOI, there was still a small percentage of rAAV2–LacZ-transduced SKOV-3ip, made evident by a low rate of blue-stained cells (data not shown). However, the RGD-modified rAAV2 (A520/N584RGD-rAAV2) showed significant and sufficient gene transduction in the rAAV2-resistant cell line SKOV-3ip (P<0.01; Figure 2). As seen in Figure 2, A520/N584RGD–rAAV2 not only reversed the resistance of SKOV-3ip to rAAV2 gene transfer, but also transduced OVCAR-3 cells as efficiently as rAAV2.


HSPG and integrins are highly associated with rAAV2 and A520/N584RGD–rAAV gene transfer, respectively As discussed in a previous paper, A520/N584RGD–rAAV2 is a novel RGD peptide-modified rAAV2 with depleted HSPG binding ability and alternative integrin interactivity[20]. Therefore, to further characterize and compare modified/non-modified rAAV2 gene transduction on ovarian cancer cells, the expressions of HSPG and integrins ανβ3 and ανβ5 were analyzed by flow cytometry. As already known, rAAV2 binds to cells primarily via the interaction between the viral capsid and cellular surface HSPG. Viral internalization is facilitated by the presence of the coreceptor αν integrin family[13]. However, the rAAV2 capsid does not bind directly to these coreceptors[8,13]. We tested the expressions of HSPG and integrins ανβ3 and ανβ5 on ovarian cancer cell lines OVCAR-3 and SKOV-3ip in an attempt to correlate these molecular expressions with rAAV2 gene transduction efficiency. Flow cytometric profiles of the different cell lines were obtained by using an anti-HSPG antibody (HepSS-1) and anti-ανβ3 and ανβ5 antibodies (LM609 and P1F6, respectively). As shown in the results, SKOV-3ip expressed low HSPG, and OVACR-3 expressed moderate-to-high HSPG. OVCAR-3 and SKOV-3ip expressed moderate-to-high level of integrins ανβ3 or/and ανβ5 (Figure 1B, 1C, 1E, 1F). The data clearly demonstrated that there was a variation of expressions for HSPG and integrins ανβ3 and ανβ5 on different ovarian cancer cell lines. Not surprisingly, the HSPG expression level on ovarian cancer cell lines was highly correlated with susceptibility to wt-rAAV2 gene transfer, suggesting that wt-rAAV2-mediated gene transduction was highly associated with the HSPG expression level, which was consistent with previous studies[15,24]. However, there was no HSPG dose-dependent wt-rAAV2 gene transduction from our previous study. There is no evidence showing the expressions ανβ3 and ανβ5 correlating with wild-type capsid rAAV2-mediated gene transduction on ovarian carcinoma cell lines, which was confirmed by previous work on Raji and K562[8]. However, A520/N584RGD–rAAV2 transduced ovarian cancer cells independent of HSPG expression, but dependent on integrin expression. Moreover, a higher integrin expression in SKOV-3ip than OVCAR-3 was accompanied by increased gene transduction, as shown in Figure 2. Furthermore, there was extremely low integrin expression in OV3 with a low expression level for HSPG (Figure 5A, 5B). To evaluate the gene transduction associated with receptor expression, we further determined the rAAV2 gene transduction on OV3 cells, which have low expression levels for HSPG and integrins. As shown in Figure 5D, both wt-rAAV2–GFP and A520/N584RGD–rAAV2–GFP showed low transduction in OV-3 cells in the absence of estradiol, and there was no significant difference. These data confirmed that rAAV2-mediated gene transduction was closely related with viral attachment and internalization.

Estrogen stimulates certain ovarian cancer cell proliferation It has been reported that estrogen plays an important role in ovarian cancer occurrence, progress, metastasis, and treatment. Different responses to estrogen can predict the prognosis of ovarian cancer treatment and prognosis. Therefore, anti-estrogen treatment should be individualized. First, we evaluated the estrogen receptor expression on the tested cell lines OVCAR-3 and SKOV-3ip. The flow cytometric analysis showed that these 2 cell lines express estrogen receptor α and β (data not shown). We further investigated the cell proliferation of ovarian cancer cell lines in response to estradiol. Ovarian cancer cells were grown in hormone-free culture medium to clarify the steroid hormone background at least 3 d before estradiol experiments. Our results illustrated that 17β-estradiol-incubated SKOV-3ip proliferated quicker compared to estradiol-free SKOV-3ip, as shown in Figure 3 (P<0.05). However, there was no visible cell proliferation alternation on the estradiol-treated OVCAR-3 cells, as shown in Figure 3 (P>0.05). This indicated that there was a varied response to 17β-estradiol among different ovarian carcinoma cells, which may be due to estrogen receptor variation[7,25].

17β-Estradiol augmented A520/N584RGD–rAAV2 gene transduction in ovarian cancer cells Some ovarian cancers secrete sex hormones, such as estrogen and progesterone. Estrogen was reported to stimulate some ovarian cancer cell proliferation. High estrogen levels were considered risk factors for ovarian cancer, and anti-estrogen treatment was recommended to the ovarian cancer patient. As discussed earlier, SKOV-3ip proliferated quicker in the presence of estradiol. However, OVCAR-3 did not exert a visible response in cell proliferation towards to estradiol. We then determined the influence of estradiol on gene transduction in OVCAR-3 and SKOV-3ip. Our data clearly documented that wt-rAAV2 gene transduction efficiency significantly decreased from 35.6% of estradiol-free OVCAR-3 to 25.6% of estradiol-incubated OVCAR-3, as presented in Figure 2 (P<0.05). A520/N584RGD–rAAV2 gene transduction did not show a significant change in OVCAR-3 when it was either incubated with estradiol or estradiol free (P>0.05; Figure 2). However, A520/N584RGD–rAAV2 gene transduction was profoundly enhanced in estradiol-incubated SKOV-3ip cells compared to the estradiol-free cells (P<0.05; Figure 2). Moreover, as shown in Figure 5D, there was significant GFP gene transduction enhancement in estradiol-cultured OV-3 cells exposed to A520/N584RGD–rAAV2–GFP compared to wt-rAAV2–GFP.
17β-Estradiol influenced the expressions of HSPG and integrins ανβ3 and ανβ5 In the present study, we demonstrated the increased A520/N584RGD–rAAV2 gene transduction and decreased wt-rAAV2 gene transduction in the presence of estradiol; however, the molecules associated with altered AAV2 gene transduction need to be further analyzed. Estradiol was shown to stimulate certain ovarian cancer cell proliferation. Strong proliferation activity is a characteristic of cancer cells, and integrins are more frequently upregulated in the transformed cells. Estradiol was reported to aggravate the ovarian cancer cells, depending on the estrogen receptor expression level. We determined OVCAR-3 and SKOV-3ip both expressed the estrogen receptor. Thereafter, we further examined the changes of the expressions of HSPG and integrins ανβ3 and ανβ5 on 17β-estradiol-challenged SKOV-3ip and OVCAR-3. There was a detectable HSPG expression reduction (50%) and integrin ανβ5 expression increased almost 4-fold on SKOV-3ip (P<0.01; Figure 4). For OVCAR-3, there was a slight alteration of both integrins and HSPG, as seen in Figure 4. A520/N584RGD–rAAV2 gene transduction was observed to correspondingly increase on 17β-estradiol-challenged SKOV-3ip, but not OVCAR-3. These data revealed that the integrin ανβ5 expression played an important role for A520/N584RGD–rAAV2 attachment and internalization. Our findings show that 17β-estradiol downregulates wt-rAAV2 gene transduction on ovarian cancer cell OVCAR-3, but does not decrease A520/N584RGD–rAAV2 gene transduction in OVCAR-3 and SKOV-3ip. Like SKOV-3ip, the increased integrin expression on OV3 cells exposed to estradiol, and enhanced gene transduction mediated by A520/N584RGD–rAAV2–GFP was also observed, as shown in Figure 5. This is in accordance with a previous study on the wt-rAAV2-reduced gene expression in female primates in vivo[16].

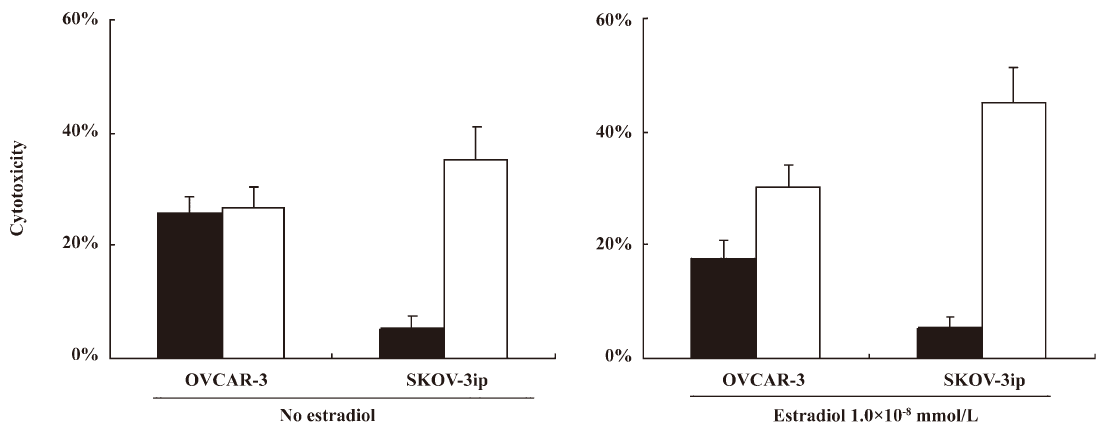
17β-Estradiol augmented A520/N584RGD–rAAV–HSV-tk-mediated ovarian cancer cell death After clarification of enhanced A520/N584RGD–rAAV2 gene transduction in the estradiol environment, we observed the GCV sensitivity of ovarian cancer SKOV-3ip transduced by A520/N584RGD–rAAV–HSVtk. To observe the antitumoral effect of rAAV2 on ovarian cancer cells, rAAV2–HSV-tk vectors and viral vectors were constructed. We further investigated the cell death caused by these rAAV–HSV-tk vectors in the presence of GCV in ovarian cancer cells. Cells were treated similarly as gene transduction experiments. The results turned out that RGD-modified rAAV2 transduced ovarian cancer sufficiently, and interest gene expression mediated cancer cell death, as shown in Figure 6 (P<0.05). A520/N584RGD–rAAV–HSV-tk exerted a profound, cytotoxic effect on ovarian cancer cells in the presence of estradiol. A520/N584RGD–rAAV2 interacted with integrins specifically, independent of heparin sulfate, which has been confirmed previously[20]. Estrogen was shown to stimulate SKOV-3ip proliferation, and A520/N584RGD–rAAV–HSV-tk exerted more significant anticancer capacity in estrogen-cultured SKOV-3ip than estrogen-free cells. These data strongly suggest that A520/N584RGD–rAAV2 transduces estrogen-incubated SKOV-3 more efficiently than estrogen-free cells. Therefore, A520/N584RGD–rAAV–HSV-tk-transduced cells express higher HSV-tk, along with an increased standby effect, which made cells more susceptible to GCV. A more significant tumor-inhibitory effect was observed in A520/N584RGD–rAAV–HSV-tk-transduced estrogen-cultured SKOV-3ip cells.
Discussion
In the present study, we found that the RGD peptide-modified rAAV2 achieved greater gene transduction on HSPG deficiency ovarian cancer cells than wild-type capsid rAAV2. More significantly, this RGD-modified rAAV2 even achieved a more profound interest gene expression in ovarian cancer cells in the presence of estrogen. However, the wild-type capsid rAAV2 not only had limited gene delivery to different ovarian cancer cell lines, but also failed to successfully transduce ovarian cancer cells in the presence of estrogen, which is close to the in vivo ovarian cancer environment. These discoveries implied that rAAV2 with the RGD-modified capsid might be a better gene therapy vector for ovarian cancer in the future. Estrogen was found to not only upregulate integrin expression on ovarian cancer cells, but also decrease HSPG level, which further suggested that RGD-modified rAAV2 might be a better gene vector for ovarian cancer gene therapy over wt-rAAV2 in vivo.
It is widely considered that AAV2-mediated gene transduction is highly related to HSPG expression, and coreceptor integrins and fibroblast growth factor receptor (FGFR) are also involved in gene delivery. Heparin sulfate proteoglycan is the receptor of AAV2, a key factor for rAAV infection, which is widely expressed on the cell membrane. However, there are still many cells that express low or no HSPG, which make them resistant to AAV2 infection, such astrocytes, some leukemia cell lines, such as K562 and Raji[9], and certain ovarian cancer cell lines. High HSPG-expressing cells are sensitive to rAAV2 infection, while low-expressing or no expression ones are resistant. Our study and those of others have revealed that the HSPG expression varies greatly on ovarian cancer cell lines and human ovarian cancer tissue[15]. Integrin expression generally increases on transformed cells, as shown in ovarian cancer cells, which makes integrins a possible target marker for gene therapy on ovarian cancer and other tumors[26–29]. Integrin ανβ5 is a coreceptor for the adeno-associated virus, which helps viral internalization[13]. The use of chelating agent EDTA to disrupt the integrins can block the gene transduction mediated by AAV, which confirms that integrins plays an important role in the process of AAV attachment and internalization. However, integrins can not initiate the gene delivery of wt-rAAV2, as evidenced by the resistance of SKOV-3ip and Hey to wt-rAAV2[9]. HSPG and integrins are critical factors for wt-rAAV2 infection.
Heparin sulfate is the major binding site for AAV2, and the regulation of this molecule can affect AAV2 gene transduction significantly. Soluble heparin almost completely blocks AAV2 gene transduction at proper concentrations in vitro. The heparin sulfate level on the cell membrane was found to be reduced on the cell surface in the presence of estradiol, which was associated with low gene transduction. In this paper, we chose OVCAR-3 and SKOV-3ip for their gene expression differences in HSPG and integrin expressions. HSPG in estradiol-incubated OVCAR-3 was greatly reduced, clearly indicating that estradiol affected the membrane HSPG level. Heparin sulfate inversion could be the explanation for this detectable and reduced HSPG level by flow cytometry, and it was previously reported that estrogen promoted heparin sulfate inversion. Therefore, this inversion affects the AAV viral attachment and internalization, which may also be the cause of low transduction in female mice compared to male mice in vivo.
Estrogen not only affects the heparin sulfate level on ovarian cancer cells, but also stimulates cell proliferation and the expressions of other molecules. Ovarian cancer cell SKOV-3ip showed higher proliferation ability after the incubation of estradiol. As reported in other studies, estrogen could be a stimulator for ovarian cancer cells, and a high estrogen level could be a risk factor for ovarian cancer. Furthermore, anti-estrogen treatment is a routine therapeutic strategy for ovarian cancer patients. However, not all ovarian cancers respond to estrogen in this pattern, as shown in clinical status. From our in vitro cell line experiment, the data presented here indicates that there is a varied response to estrogen for ovarian cancer cells. High-level of estrogen may inhibit wt-rAAV2 gene transduction, as evidenced by reduced membrane HSPG. It is to our surprise that A520/N584RGD–rAAV2 obtained even higher gene transduction in estradiol-incubated SKOV-3ip than estradiol-free SKOV-3ip. Interestingly, we found that the αυβ3 integrin expression increased on SKOV-3ip when stimulated with 17β-estradiol, but for the OVCAR-3 cell line, there was a decrease of membrane HSPG accompanied by reduced gene transduction of AAV2 on OVCAR-3 challenged with 17β-estradiol. Heparan sulfate (HS) proteoglycans are essential for and are positively correlated with AAV2 transduction efficiency, whereas integrins are not critical for AAV2 gene transduction on ovarian cancer cell lines.
17β-Estradiol is regarded as an estrogen which stimulates cell growth, transformation, and proliferation on ovarian cancer cells[30–32]. A prospective study showed that estrogen replacement therapy increased the ovarian cancer mortality as well as ovarian cancer morbidity[32]. Up to 60% of ovarian cancer or recurrent ovarian cancer patients are estrogen receptor positive, and estrogen may necessarily cause tumors to grow or reappear. Estradiol can increase cell numbers of ovarian cancer cell lines (DOV13 and SKOV-3)[6]. A paradoxical report shows that estrogen can inhibit the occurrence of ovarian cancer and tumor metastasis[25]. In our study, not all ovarian cancer cells responded to estradiol by proliferation, which could be because the estrogen receptor (ER) expression level was as confirmed as clinical (ER)+ or ER– ovarian cancer[25]. The proliferation response of some ovarian cancer cell lines, such as SKOV-3, was observed, while there was no proliferation response on OVCAR-3. Ovarian cancer cells respond differently to estrogen, such as estradiol, which is closely related with the estrogen receptor expression level and other possible factors. For estrogen receptor-positive patients, ovarian tumor cells respond to estrogen and are sensitive to estrogen therapy. We found SKOV-3ip to be responsive to estrogen stimulation, as evidenced by cell growth and proliferation. Not surprisingly, there were no significant integrin expression changes on OVCAR-3 either, which implied that the upregulation of the integrin expression and proliferation may be associated with estrogen receptor signaling. SKOV-3ip expresses higher estrogen receptor α than β, while OVCAR-3 generates higher estrogen receptor β than α, which might explain the different responses of cell proliferation and integrin expression towards estradiol. Estrogen is a risk factor for ovarian cancer by inducing transformation of the tumor cells, which was proved by up-regulation of tumor marker integrins. Furthermore, estradiol stimulation upregulated the integrin expression. The increased integrin expression has been often determined on transformed cells, which implies estradiol’s involvement in the oncogenesis of ovarian cancer[33].
The expressions of HSPG and integrin β3 and β5 have been analyzed to observe the difference between response cell lines. It has been reported that 17β-estradiol could turn over the cell surface HSPG[18], which in turn may reduce the rAAV2 attachment to the cell membrane. Accordingly, gene transduction could be reduced. In our study, there was a gene transduction reduction after the OVCAR-3 ovarian cancer cells were incubated with 17β-estradiol, which is in accordance with other in vivo studies[16,17]. However, for estradiol-incubated SKOV-3ip, there was no detectable reduction of gene transduction, which may be due to the low HSPG expression on SKOV-3ip, as evidenced by the HSPG and integrin expression profiles.
We have demonstrated that A520/N584RGD–rAAV2 can deliver genes to target cells independent of HSPG. It was proved by various cell incubation environment. Furthermore, the RGD peptide can counteract its gene transduction, as previously described. We previously reported that the RGD peptide can inhibit the gene transduction mediated by RGD-modified rAAV[9,15,20]. Similar data about RGD-4C insertion in adenoviruses also confirmed that this peptide can interact with integrins specifically[3,28,29]. All the data reported here strongly revealed that the RGD–integrin interaction was involved in the process of gene transduction mediated by rAAV. For the transformed cells that highly express integrins, RGD–rAAV2 is an ideal tool for gene delivery.
As discussed earlier, integrin can help the internalization and trafficking of AAV particles in the cytoplasm. Estrogen, such as 17β-estradiol, not only promotes heparin sulfate reverse on the membrane, but also upregulates integrin expression on certain types of ovarian cancer cell lines. Moreover, many estrogen receptor-positive ovarian cancer individuals have estrogen-positive serum, which may stimulate their integrin expression in vivo. All of these findings make RGD peptide-modified AAV2 more applicable and practical for ovarian cancer tumor therapy.
Taken together, our study implies that RGD-modified rAAV2 can efficiently transduce ovarian cancer cells, and RGD-modified rAAV2 has great potential in ovarian cancer gene therapy in vivo in the future.
Acknowledgements
The authors are grateful to the Vector Core Laboratory at the Children’s Research Center, Children’s Hospital (Columbus, OH, USA), and to Dr KP CLARK for technical assistance. We thank Xin-yuan LIU from the Shanghai Institute of Cell Biology and Biochemistry (Shanghai, China) for guidance on the research work, and Meng-chao WU and Dr Qi-jun QIAN from the Eastern Hepatobiliary Surgical Hospital (Shanghai, China) for strong support and scientific discussion.
References
- Sandalon Z, Bruckheimer EM, Lustig KH, Rogers LC, Peluso RW, Burstein H. Secretion of a TNFR:Fc fusion protein following pulmonary administration of pseudotyped adeno-associated virus vectors. J Virol 2004; 78: 12 355–65.
- Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update 2006;12:385-400.
- Lam JT, Kanerva A, Bauerschmitz GJ, Takayama K, Suzuki K, Yamamoto M, et al. Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy. J Gene Med 2004;6:1333-42.
- Behbakht K, Benjamin I, Chiu HC, Eck SL, Van Deerlin PG, Rubin SC, et al. Adenovirus-mediated gene therapy of ovarian cancer in a mouse model. Am J Obstet Gynecol 1996;175:1260-5.
- Zhang HG, Xie J, Dmitriev I, Kashentseva E, Curiel DT, Hsu HC, . Addition of six-His-tagged peptide to the C terminus of adeno-associated virus VP3 does not affect viral tropism or production. J Virol 2002; 76: 12 023–31.
- Song S, Lu Y, Choi YK, Han Y, Tang Q, Zhao G, et al. DNA-dependent PK inhibits adeno-associated virus DNA integration. Proc Natl Acad Sci USA 2004;101:2112-6.
- Young SM Jr, Samulski RJ. Adeno-associated virus (AAV) site-specific recombination does not require a Rep-dependent origin of replication within the AAV terminal repeat. Proc Natl Acad Sci USA 2001; 98: 13 525–30.
- Shi GX, Wang Y, Liu Y, Cui W, Zhao FT, Zhu LP, et al. Long-term expression of a transferred gene in Epstein-Barr virus transformed human B cells. Scand J Immunol 2001;54:265-72.
- Shi W, Bartlett JS. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol Ther 2003;7:515-25.
- Bartlett JS, Kleinschmidt J, Boucher RC, Samulski RJ. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab’gamma)2 antibody. Nat Biotechnol 1999;17:181-6.
- Ponnazhagan S, Mahendra G, Kumar S, Thompson JA, Castillas M Jr. Conjugate-based targeting of recombinant adeno-associated virus type 2 vectors by using avidin-linked ligands. J Virol 2002; 76: 12 900–7.
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A, et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med 1999;5:71-7.
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998;72:1438-45.
- Vermeij J, Zeinoun Z, Neyns B, Teugels E, Bourgain C, De Grève J, et al. Transduction of ovarian cancer cells: a recombinant adeno-associated viral vector compared to an adenoviral vector. Br J Cancer 2001;85:1592-9.
- Shi W, Hemminki A, Bartlett JS. Capsid modifications overcome low heterogeneous expression of heparan sulfate proteoglycan that limits AAV2-mediated gene transfer and therapeutic efficacy in human ovarian carcinoma. Gynecol Oncol 2006;103:1054-62.
- De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther 2006;13:67-76.
- Dodge JC, Passini MA, Song A, O’Riordan CR, Cheng SH, Stewart GR. Sex and estrous cycle stage influence the efficiency of AAV-mediated gene transfer in the rodent brain. Mol Therapy 2005;11:192.
- Morris JE, Potter SW, Gaza-Bulseco G. Estradiol-stimulated turnover of heparan sulfate proteoglycan in mouse uterine epithelium. J Biol Chem 1988;263:4712-8.
- Huser D, Heilbronn R. Adeno-associated virus integrates site-specifically into human chromosome 19 in either orientation and with equal kinetics and frequency. J Gen Virol 2003;84:133-7.
- Shi X, Fang G, Shi W, Bartlett JS. Insertional mutagenesis at positions 520 and 584 of adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors with eliminated heparin- binding ability and introduced novel tropism. Hum Gene Ther 2006;17:353-61.
- Stachler MD, Bartlett JS. Mosaic vectors comprised of modified AAV1 capsid proteins for efficient vector purification and targeting to vascular endothelial cells. Gene Ther 2006;13:926-31.
- Ruffing M, Heid H, Kleinschmidt JA. Mutations in the carboxy terminus of adeno-associated virus 2 capsid proteins affect viral infectivity: lack of an RGD integrin-binding motif. J Gen Virol 1994;75:3385-92.
- Schnepp BC, Jensen RL, Chen CL, Johnson PR, Clark KR. Characterization of adeno-associated virus genomes isolated from human tissues. J Virol 2005; 79: 14 793–803.
- Opie SR, Warrington KH Jr, Agbandje-McKenna M, Zolotukhin S, Muzyczka N. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J Virol 2003;77:6995-7006.
- Hayashido Y, Lucas A, Rougeot C, Godyna S, Argraves WS, Rochefort H. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int J Cancer 1998;75:654-8.
- Van Houdt WJ, Wu H, Glasgow JN, Lamfers ML, Dirven CM, Gillespie GY, et al. Gene delivery into malignant glioma by infectivity-enhanced adenovirus: in vivo versus in vitro models. Neuro Oncol 2007;9:280-90.
- Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther 2007;18:589-602.
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst 2007;99:1410-4.
- Dehari H, Ito Y, Nakamura T, Kobune M, Sasaki K, Yonekura N, et al. Enhanced antitumor effect of RGD fiber-modified adenovirus for gene therapy of oral cancer. Cancer Gene Ther 2003;10:75-85.
- Kaplitt MG, Makimura H. Defective viral vectors as agents for gene transfer in the nervous system. J Neurosci Methods 1997;71:125-32.
- Qing K, Bachelot T, Mukherjee P, Wang XS, Peng L, Yoder MC, et al. Adeno-associated virus type 2-mediated transfer of ecotropic retrovirus receptor cDNA allows ecotropic retroviral transduction of established and primary human cells. J Virol 1997;71:5663-7.
- Rodriguez C, Patel AV, Calle EE, Jacob EJ, Thun MJ. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA 2001;285:1460-5.
- Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res 2001;61:6768-76.
