Pharmacokinetic and toxicity study of intravitreal erythropoietin in rabbits1
Introduction
Apoptosis is an important mode of cell death in diabetic retinopathy[1,2]. One of the promising agents, erythropoietin (EPO), a 34-kDa glycoprotein hormone, has been reported as a cytoprotectant for prevention and treatment of early diabetic retinopathy (EDR) in diabetic rats[3]. Accumulating data have demonstrated that EPO displays effective neuroprotective properties in a spectrum of different animal models including several degenerative conditions as well as toxic insults[4,5].
The levels of EPO receptor (EpoR) increase in detached retina[6] and EDR[3]. IVit of EPO maintains the blood-retina barrier (BRB) and protects retinal neurons from apoptosis in early diabetes[3]. IVit of EPO had a protective effect on retinal ganglion cell (RGC) in a rat glaucoma model[7], and significantly increased RGC somata and axon survival following optic nerve transection[8], indicating its neuroprotective and neurotrophic effects.
The pharmacokinetics of EPO were studied in rabbits as well as in other species after systemic administration[9,10]. However, the toxicity of the IVit of EPO has not been studied, and the pharmacokinetic profile after IVit is also currently unknown. Therefore, in the present study, the ocular and systemic toxicity and pharmacokinetic profile after IVit of EPO were studied in New Zealand albino rabbits.
Materials and methods
Animals 104 male New Zealand albino rabbits, weighing 2.5–3.0 kg, were included in the study. All of the experimental procedures adhered to the ARVO Resolution on the Treatment of Animals in Research. For the toxicity study, before IVit or ocular examinations, the rabbits were anesthetized by an intravenous injection of 1.5–2.0 mL of 3% sodium pentobarbital. Topical anesthesia with 0.4% oxybuprocaine hydrochloride eye drops (Eisai Co Ltd, Tokyo, Japan) was applied to reduce the animals’ discomfort. The pupils were fully dilated with 0.5% tropicamide (Wuxi Shanhe Group, Jiangsu, China). A complete eye examination including electroretinogram (ERG), and fluorescein angiography (FA) was carried out before and after the IVit. For histological evaluation, 6 rabbits at each time point were killed at 2 weeks, 4 weeks or 6 months after IVit, by intravenous injection of an overdose of sodium pentobarbital. For the pharmacokinetic study, 4 rabbits at each time point were killed from 0 h to 14 d after EPO IVit.
Erythropoietin Recombinant human EPO (rHuEPO, 1×107 U/L in the original vial, Shenyang Sunshine Pharmaceutical Co, Ltd, Shenyang, China), diluted by normal saline (NS), was used in the present study. A single injection (1000 U/100 µL per eye, 100 U/50 µL per eye or 10 U/50 µL per eye) or monthly repeated EPO injections (0.6 U/50 µL per eye, 6 times) were carried out to the left eyes of the rabbits. The dose of 0.6 U/eye was the therapeutic level in rabbits, calculated based on the rat study of EDR as reported previously[3]. The right eyes were injected with the same volume of NS as a control. For the pharmacokinetic study, EPO (5 U/50 µL per eye), was given intravitreally into the left eyes, whereas the right eyes were left untreated. The optimal dose range of intravitreal EPO for its protection in diabetic retina of rat is 8–32 mU/eye[3]. The optimal doses of EPO in possible human objects were calculated based on the optimal doses obtained from the rat study. The vitreous volumes for rat and human are about 15 µL and 4.5 mL, respectively. The optimal doses for EPO in human are estimated as 2.4–9.6 U/eye. Therefore, we choose 5 U/eye of EPO as the designed therapeutic dose for human objects. The same dose (5 U/eye) was used for pharmacokinetic study in rabbits, although the vitreous volume of rabbit is about one third of the human.
Intravitreal injection The IVit was carried out, using a 30-gauge, 0.5-inch needle (BD Biosciences, Franklin Lakes, NJ, USA) on a microsyringe (Hamilton, Reno, NV, USA), at approximately 2 mm posterior to the limbus in the superotemporal quadrant. Povidone-iodine solution (5%) was applied before and immediately after the IVit.
Slit lamp and fundus examination Before and after IVit, the anterior segments were examined and photographed through a slit-lamp with an anterior segment digital imaging system (YZ5F Slit lamp; 66 Vision Tech, Co, Ltd, Suzhou, China and YTFX-QB1A Imaging System, Xiamen, China). After 1 d, 3 d, 1 week, 2 weeks, 1 month and 6 months following the IVit, fundus examination was carried out by indirect ophthalmoscopy with a 20-D aspheric lens. Fluorescein sodium injection (20%, Guangzhou Mingxing Pharmaceutical Co, Ltd, Guangzhou, China) was applied via the marginal ear vein. FA was carried out in randomly selected animals (3 in each group) at intervals of 2 weeks, 4 weeks and 6 months, following the IVit.
Electroretinogram Retinal neuron functions were examined with ERG, using a Tomey EP-1000 system (Japan). After the pupils were dilated, the rabbits were dark-adapted for at least 30 min before the examination. Flash ERG responses were recorded from both eyes through corneal electrodes (Fabrinal SA, Switzerland), with the negative electrode placed in the subcutaneous space of the forehead, and the ground electrode clipped to the earlobe. Electric gel was used to maintain the proper conduction. The ERG signals were amplified (20 000×) and filtered (0.1–300 Hz) by differential amplifiers. Light stimuli were obtained from a Ganzfeld light source with a maximum intensity of 3 cd·s/m2. ERG analysis was based on the b-wave amplitude, measured from the trough of the a-wave to the peak of the b-wave.
Histological examination At the designed time points, rabbits were killed. The eyes were enucleated and fixed in phosphate-buffered saline (PBS)-buffered 4% paraformaldehyde for 24 h. The orientation was marked at 12 clock of the limbus. The eyes were embedded in paraffin, sectioned at 2 µm, and stained with hematoxylin and eosin for light microscopy. The thicknesses of different retinal layers were measured on the sections that were through the optic nerve head, under 200× magnification, including: (1) outer limiting membrane to inner limiting membrane (OLM-ILM); (2) outer nuclear layer to outer plexiform layer (ONL-OPL); (3) inner nuclear layer (INL); and (4) inner plexiform layer (IPL). Two measurements were taken on each section, 1 mm distant from the edges of the optic nerve head. Data from 5 to 6 rabbits were averaged to avoid potential anatomic variations in the different topographic regions. The cell numbers in ONL and INL were counted in the same region as the thickness measurement, under the 1000× magnification. All the cell nuclei within a fixed 50 µm column, centered with the 1 mm reference lines, were counted. The cell density was expressed as the cell count/mm width of retina.
Enzyme-linked immunosorbent assay for EPO measurement At each time point the undiluted aqueous humor was collected. The vitreous samples were transferred to a tube and centrifuged at 16 000×g for 5 min at 4 °C. Supernatants were frozen at –80 °C. Blood samples were collected and then serum samples were prepared. Retinas were carefully isolated and frozen at –80 °C. Before analysis, the retina was homogenized in ice-cold radioimmune precipitation assay (RIPA) buffer supplemented with a protease inhibitor PMSF (Shenergy Bicolor Bioscience Technology Company, Shanghai, China) and sonicated at 0.5 Hz for 40 s (50-watt sonicator, Sonics & Materials, Danbury, CT, USA). The lysate was centrifuged at 20 000×g for 15 min at 4 °C. The supernatants were collected independently and frozen at –80 °C. EPO concentration was determined with an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Genetimes Technology, Inc, Shanghai, China) following the manufacturer’s protocol. Absorbance at 450 nm and reference at 600 nm was measured using a microplate reader (Safire2, Tecan Group Ltd, Maennedorf, Switzerland). The protein levels of EPO in the retina were normalized by the total protein content, as determined by a bicinchoninic acid (BCA) kit (PIERCE, IL, USA). Data are presented as U/g·protein.
Pharmacokinetic analysis Erythropoietin concentrations in the vitreous, aqueous humor, serum and retina were analyzed with ELISA as previously described. The pharmacokinetics of EPO in vitreous, aqueous humor, retina, and serum after intravitreal administration were determined by a noncompartmental method, and analyzed using the software Pharsight’s WinNonlin version 5.2 (InforSense, Shanghai, China). Values below the minimal detecting limit of the assays were excluded from all analyses.
Statistics Data are expressed as mean±SD. The statistical analyses were carried out using Student’s t-test. A P-value of 0.05 or less was considered statistically significant.
Results
Slit lamp and fundus examination At all time points, the conjunctiva and cornea were apparently normal in rabbits after IVit of EPO or NS. No anterior chamber reaction was found. No iris abnormality, particularly no rubeosis, was observed in any eye. The lens remains clear in all animals.
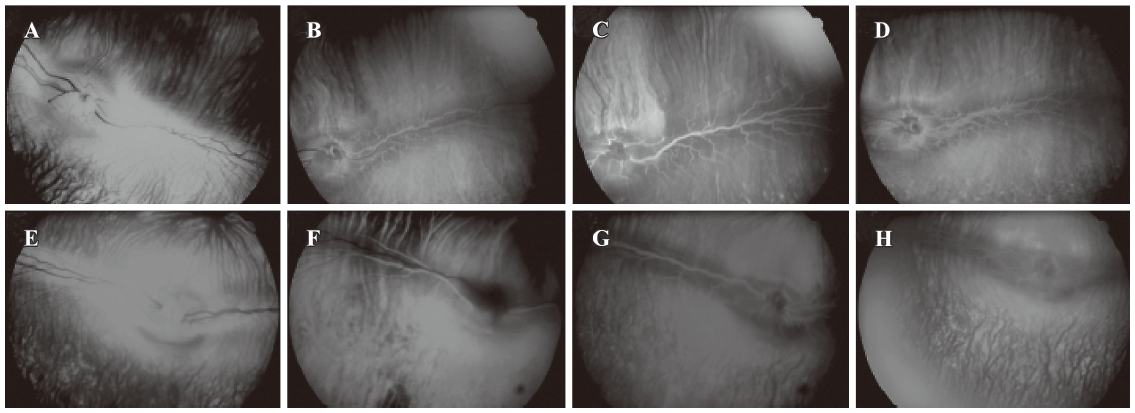
After IVit, vitreous, retina and choroid appeared to be normal through fundus photography in all 4 groups of animals (Figure 1). FA showed no sign of neovascularization, and no changes in retinal vascular leakage and choroidal perfusion up to 6 months after high doses of EPO (10 U/eye to 1000 U/eye) (Figure 2). There was no sign of vascular leakage or neovascularization after repeated EPO injections (0.6 U/eye, 6 times) (data not shown).
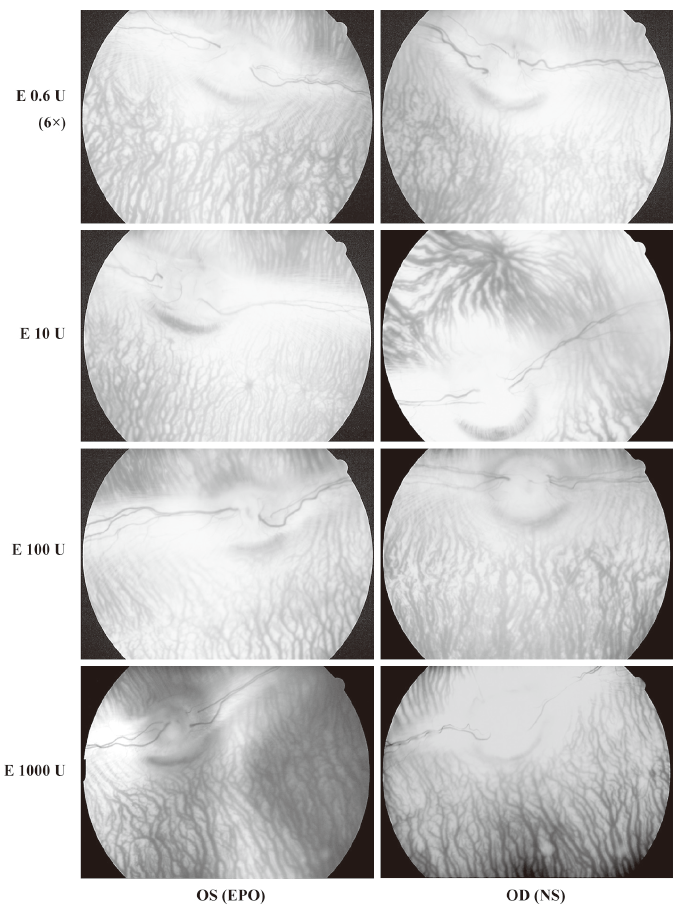
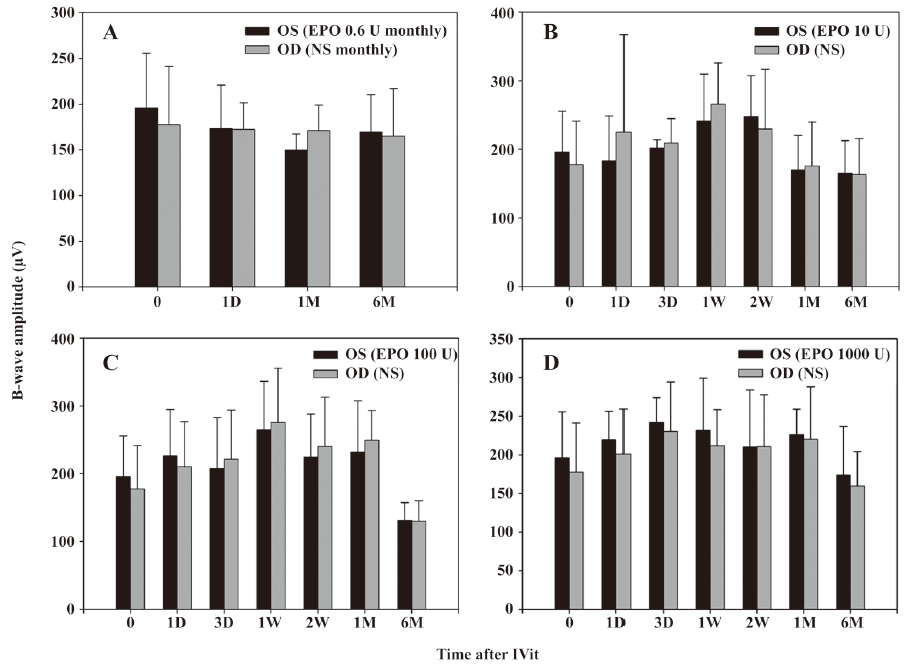
Electroretinogram Electroretinogram was carried out as an objective assessment of the retinal function of rabbit eyes. In both the single injection (10–1000 U/eye) groups and the repeated injection group, there were no significant changes in the amplitude of b-waves (P>0.05). No statistically significant change was found in b-wave amplitudes from 1 d to 6 months after IVit in any group for both eyes (Figure 3).
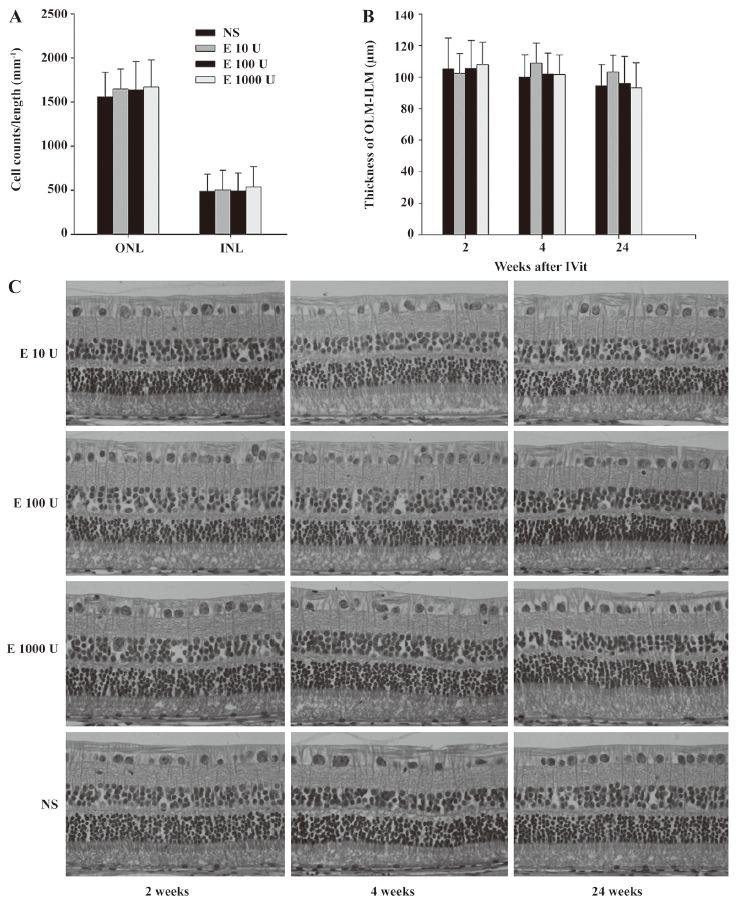
Histological examination Retinal cell counts in both ONL and INL and retinal layer thickness were essentially unchanged in different groups (Figure 4A, 4B) at 2 weeks, 4 weeks and 6 months after the IVit. No sign of retinal necrosis, cystic degeneration, or hypocellularity of the nuclear layers was observed in any of the injected groups (Figure 4C). There is no significant difference in histological examination between repeated EPO- and NS-injected eyes (data not shown).
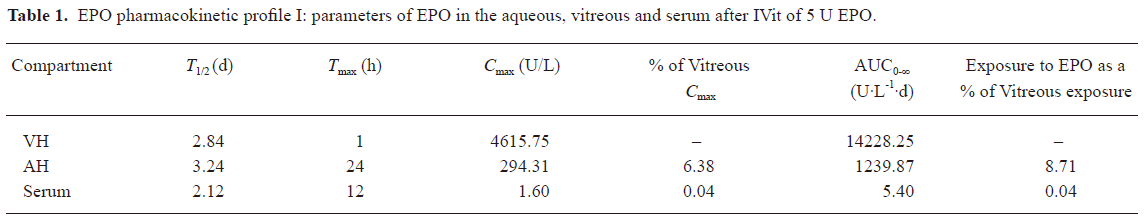
Pharmacokinetics The changes of EPO concentration over the time following IVit in vitreous, retina, aqueous humor as well as in the serum are illustrated in Figure 5. Endogenous EPO was undetectable in above compartments of the normal rabbits, because the ELISA kit is for detection of rHuEPO. After IVit of 5 U EPO, the peak concentration in vitreous was 4615.75 U/L at 1 h, and then declined in a monoexponential fashion with a half-life of 2.84 d. Vitreous EPO concentration was maintained at 155.55 U/L 2 weeks after the EPO IVit. In comparison, EPO concentration in the aqueous humor was much lower. The peak EPO concentration in aqueous humor was 294.31 U/L at 24 h after the injection. Very low concentrations of EPO were detected in the serum after IVit. A maximum serum concentration of 1.60 U/L reached at 12 h. There was no measurable EPO in the circulation 3 d after the IVit. The elimination of EPO from the aqueous humor and serum was parallel to that from the vitreous humor, with half-life times of 3.24 d (aqueous humor) and 2.12 d (serum) respectively. EPO in the retina of the injected eye reached a peak concentration of 1.36 U/g·protein at approximately 6 h after the injection. No EPO was detected in the aqueous humor or the vitreous in the non-injected fellow eye of the same animals. The basic pharmacokinetic parameters of EPO in the eyes are demonstrated in Tables 1 and 2. The maximum concentration of EPO attained in the aqueous humor was 6.38% of the maximum EPO concentration in the vitreous. The maximum serum EPO concentration was only 0.04% of the maximum vitreous EPO concentration. The total exposure of the aqueous humor to EPO was 8.71% of that of the vitreous, and the total exposure of the serum to EPO was 0.04% of that of the vitreous, indicating very low systemic exposure when comparing area under the curve (AUC) values.
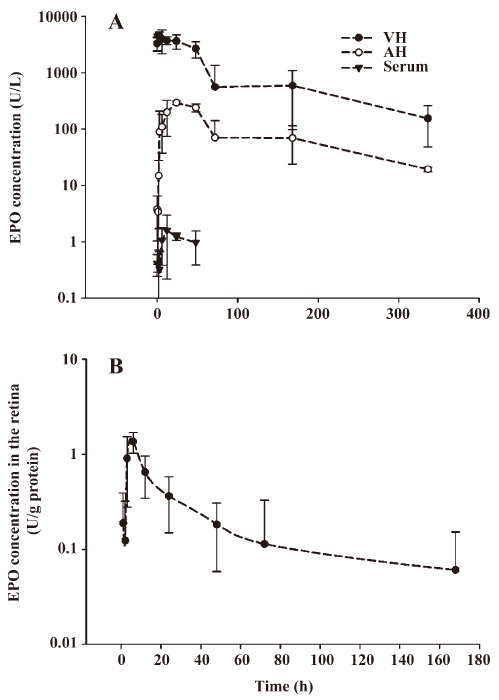
Full table
Full table
Discussion
The rHuEPO has an identical amino acid sequence to that of the isolated natural EPO. rHuEPO was approved by the Food and Drug Administration in the United States and its counterparts in other countries for the treatment of different types of anemia[11,12]. It has been reported that patients undergoing systemic EPO treatment may develop adverse reactions such as flu-like symptoms[13], seizures[14], hypertension, and increased risk of thrombotic complications[15,16]. Since systemic treatment of retinal diseases with EPO may be limited by such adverse reactions as well as from the possible erythropoietic and angiogenic activities[17,18], IVit of EPO may offer a preferred approach[3].
In the present study, a complete eye examination focusing on FA and ERG did not show any clinical and functional change to the BRB and retinal neurons after IVit of EPO in a wide range. Histological examination was also used to determine whether any structural change occurred. In the single-dose-injection experiment, the dose of 3-magnitude higher (1000 U/eye) than the designed therapeutic level (0.6 U/eye)[3] was tested to be free of side-effects. The repeated injections at the therapeutic level did not cause any change in anterior segment, vitreous or retina. For repeated higher doses of EPO (higher than 0.6 U/eye), a recent report demonstrated that monthly IVits of rHuEPO at a dose of up to 1000 U over 7 months were well-tolerated and do not cause adverse effects on retinal function, architecture, or vasculature in a rabbit model[19]. This experiment also verified the safety of monthly injections in the eyes with a wide range of EPO.
The present study provides ocular tissue distribution and clearance of EPO after IVit and lays a foundation of dose designing and of assessment of systemic safety. The rabbit system is a well-established model used for the study of intravitreal pharmacokinetics of ocular drugs[20,21]. However, limitations of the rabbit model must include that the retina is less vascular than in the human eye, and the volume of distribution is different. The half-life of EPO, when given systemically, is 4–13 h in the human[22] and about 4 h in rabbits[23]. In comparison, the half-life of EPO, when given directly into the eye, was 2.84 d in vitreous cavity and 3.24 d in aqueous humor in the rabbit. After 2 weeks, vitreous EPO was still maintained at high levels. This was not surprising because the lack of vasculature in the vitreous would significantly slow the clearance of the drug from the vitreous. The maximum serum concentration after an IVit was low, 1.6 U/L, and no EPO was found in the non-injected control eyes of the same rabbit. Both the small amount of injected EPO in the eye and the greater volume of the circulating blood, as well as the quick clearance of EPO by binding to its receptor and by degradation in rabbits may explain such observations.
In conclusion, clinical observation, electrophysiological and light microscopic evaluation demonstrated that intravitreal delivery of EPO is safe, well tolerated and nontoxic to the intraocular structures at doses from 0.6 U to 1000 U per rabbit eye. After IVit of 5 U/eye, the serum EPO levels were always minimal, within a physiologic range, indicating a rapid systemic clearance of EPO. After 2 weeks, vitreous EPO was still maintained at high levels, whereas serum EPO could not be detected. This pharmacokinetic profile is favorable for intravitreal use of EPO by episodic (such as monthly) injections.
References
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 2002;47 Suppl 2:S253-62.
- Li W, Yanoff M, Jian B, He Z. Altered mRNA levels of antioxidant enzymes in pre-apoptotic pericytes from human diabetic retinas. Cell Mol Biol (Noisy-le-grand) 1999;45:59-66.
- Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y, et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 2008;49:732-42.
- Sharples EJ, Thiemermann C, Yaqoob MM. Novel applications of recombinant erythropoietin. Curr Opin Pharmacol 2006;6:184-9.
- Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ 2004;11 Suppl 1:S37-44.
- Xie Z, Wu X, Qiu Q, Gong Y, Song Y, Gu Q, et al. Expression pattern of erythropoietin and erythropoietin receptor in experimental model of retinal detachment. Curr Eye Res 2007;32:757-64.
- Tsai JC, Wu L, Worgul B, Forbes M, Cao J. Intravitreal administration of erythropoietin and preservation of retinal ganglion cells in an experimental rat model of glaucoma. Curr Eye Res 2005;30:1025-31.
- King CE, Rodger J, Bartlett C, Esmaili T, Dunlop SA, Beazley LD. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol 2007;205:48-55.
- Jolling K, Perez Ruixo JJ, Hemeryck A, Vermeulen A, Greway T. Mixed-effects modelling of the interspecies pharmacokinetic scaling of pegylated human erythropoietin. Eur J Pharm Sci 2005;24:465-75.
- Yoon WH, Park SJ, Kim IC, Lee MG. Pharmacokinetics of recombinant human erythropoietin in rabbits and 3/4 nephrectomized rats. Res Commun Mol Pathol Pharmacol 1997;96:227-40.
- Jurado Garcia JM, Torres Sanchez E, Olmos Hidalgo D, Alba Conejo E. Erythropoietin pharmacology. Clin Transl Oncol 2007;9:715-22.
- Hanna L. Epoietin alfa (EPO) for anemia. BETA 1998;40:42.
- Goy A, Belanger C, Casadevall N, Picard F, Guesnu M, Jaulmes D, et al. High doses of intravenous recombinant erythropoietin for the treatment of anaemia in myelodysplastic syndrome. Br J Haematol 1993;84:232-7.
- Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989;111:992-1000.
- Kanbay M, Akcay A, Delibasi T, Uz B, Kaya A, Koca C, et al. Comparison of effects of darbepoetin alfa and epoetin alfa on serum endothelin level and blood pressure. Adv Ther 2007;24:346-52.
- Luft FC. Erythropoietin and arterial hypertension. Clin Nephrol 2000;53 Suppl:S61-4.
- Ribatti D, Vacca A, Roccaro AM, Crivellato E, Presta M. Erythropoietin as an angiogenic factor. Eur J Clin Invest 2003;33:891-6.
- Becerra SP, Amaral J. Erythropoietin–an endogenous retinal survival factor. N Engl J Med 2002;347:1968-70.
- Song BJ, Cai H, Gomes N, Chang S, Tsai J, Forbes M, Ocular Safety of Serial Intravitreal Injections of Recombinant Human Erythropoietin: A 7 Month Study in Rabbits [CD-ROM]. Fort Lauderdale: The Association for Research in Vision and Ophthalmology; 2008.
- Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114:855-9.
- Fauser S, Kalbacher H, Alteheld N, Koizumi K, Krohne TU, Joussen AM. Pharmacokinetics and safety of intravitreally delivered etanercept. Graefes Arch Clin Exp Ophthalmol 2004;242:582-6.
- Boelaert JR, Schurgers ML, Matthys EG, Belpaire FM, Daneels RF, De Cre MJ, et al. Comparative pharmacokinetics of recombinant erythropoietin administered by the intravenous, subcutaneous, and intraperitoneal routes in continuous ambulatory peritoneal dialysis (CAPD) patients. Perit Dial Int 1989;9:95-8.
- Sytkowski AJ, Lunn ED, Davis KL, Feldman L, Siekman S. Human erythropoietin dimers with markedly enhanced in vivo activity. Proc Natl Acad Sci USA 1998;95:1184-8.
