Effect of daidzin, genistin, and glycitin on osteogenic and adipogenic differentiation of bone marrow stromal cells and adipocytic transdifferentiation of osteoblasts
Introduction
Bone metabolism is regulated by functions of osteoblasts and osteoclasts, which are localized in bone tissues. Osteoblasts stimulate bone formation and calcification while osteoclasts promote bone resorption. Many experimental results on the action of isoflavones on the differentiation and/or function of osteoblasts and/or osteoclasts have been reported[1].
Bone marrow stromal cells (MSC) are pluripotent cells that have the capacity to differentiate into osteoblasts, adipocytes, chondrocytes, myoblasts, or fibroblasts[2,3]. Thus, lineage determination between osteoblasts and adipocytes could be a critical component in the regulatory pathways of osteoblastogenesis[4]. Consistently, an increased lipid accumulation in the bone marrow has been reported in association with age-related bone loss implying an inverse relationship between osteoblastogenesis and adipogenesis. Indeed, it is now hypothesized that an increase in the number of adipocytes occurs at the expense of osteoblasts in osteopenic disorders. Furthermore, there is more and more evidence that suggest a large degree of plasticity exists between osteoblasts and adipocytes and that this transdifferentiation is reciprocal[5]. It was reported that there was a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis[6]. Therefore, it is possible that the inhibition of marrow adipogenesis with a concomitant increase in osteoblastogenesis could provide a therapeutic target with which to either prevent further increases in adipocyte formation or divert existing adipocytes to become more osteoblasts with a resulting increase in functional bone cells[7]. Moreover, recent studies in vitro have demonstrated that 17β-estradiol suppresses the expression of lipoprotein lipase (LPL), a marker of adipocyte differentiation in an extramedullary preadipocytic cell line, 3T3L1[8]. Okazaki et al reported that 17β-estradiol directly modulated differentiation of bipotential stromal cells into the osteoblast and adipocyte lineages, promoting osteoblast differentiation and inhibiting adipocyte differentia-tion, causing a lineage shift toward the osteoblast[9]. Such effects will lead to direct stimulation of bone formation and thereby contribute to the protective effects of 17β-estradiol on bone.
So far, the effect of daidzin, genistin, and glycitin on the osteogenic and adipogenic differentiation of MSC and the adipogenic transdifferentiation of osteoblasts was not reported. The aim of the present investigation was to address the question of whether daidzin, genistin and glycitin had effects on osteogenic and adipogenic differentiation of primary mouse MSC and to investigate the adipogenic transdifferentiation of primary mouse osteoblasts compared with 17β-estradiol.
Materials and methods
Materials Kunming (KM) mice were purchased from the Guangming Weiwu Biological Product Factory (Shenzhen, China). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum were purchased from Gibco (Carlsbad, CA, USA). The 17β-estradiol, benzylpenicillin, streptomycin, MTT, β-glycerophosphate, dexamethasone, ascorbic acid, insulin, oil red O stain were obtained from Sigma Chemical Co (St Louis, MO, USA). Demethyl sulfoxide (Me2SO) was purchased from Sangon (Shanghai, China). Genistin, daidzin, and glycitin were from Shanghai TAUTO Biotech Co (Shanghai, China). An alkaline phosphatase (ALP) activity kit was obtained from Nanjing Jiancheng Biological Engineering Institute (Nanjing, China), micro-protein assay kit was purchased from Beyotime Biotechnology (Haimen, China).
Isolation and culture of primary bone MSC The mouse bone MSC were obtained from adult KM mice (4–6 weeks) using the method previously reported[10] with minor modification. Mice were killed by decapitation. Femora and tibiae were aseptically harvested, and the whole bone marrow was flushed using DMEM in a 1 mL syringe and a 25-gauge needle. The cells were collected and cultured in a culture flask. After a 3-d incubation in a 37 ºC, 5% CO2 humidified incubator, the nonadherent cells were removed from the cultures by gentle aspiration and the medium replaced with fresh DMEM. The medium was changed every 3 d in all the experiments.
Isolation and culture of primary osteoblasts The mice osteoblasts were isolated mechanically from newborn mice skull using a modification of the method previously reported[11]. Briefly, the skull (frontal and parietal bones) was dissected from KM mice, the endosteum and periosteum were stripped off, and the bone was cut into approximately 1–2 mm2 pieces and digested with trypsin (2.5 g/L) for 30 min, and the digestion was discarded. The bone was then digested twice with collagenase A (2.0 g/L) for an 1 h each time. The cells were collected and cultured in a culture flask. After being placed overnight in a 5% CO2 humidified incubator at 37 ºC, the DMEM was removed. The medium was then changed every 3 d in all the experiments.
Assessment of primary mouse bone MSC and osteoblast growth The protocol described by Mosmann was followed with some modifications[12]. Briefly, MSC (3×106 cells per well) and osteoblasts (1×104 cells per well) were plated in 96-well culture plates and cultured overnight at 37 ºC in a 5% CO2 humidified incubator. Daidzin, genistin, glycitin, and 17β-estradiol were then added to the wells at final concentrations of 1×10-8, 5×10-7, 1×10-6, 5×10-6, and 1×10-5 mol/L. Control wells were prepared with the addition of DMEM. Wells containing DMEM without cells were used as blanks. The plates were incubated at 37 ºC in a 5% CO2 incubator for 48 h. Upon completion of the incubation, stock MTT dye solution (20 µL, 5 g/L) was added to each well. After 4-h incubation, the supernatant was removed and Me2SO (100 µL) was added to solubilize the MTT. The optical density of each well was measured on a microplate spectrophotometer (Bio-Rad Model 680, Hercules, CA, USA) at a wavelength of 570 nm. The proliferation rate/% of control was calculated according to the formula: ODtreated/ODcontrol×100%.
Measurement of ALP activity The bone MSC were isolated as above. MSC (3×106 cells per well) were plated in 48-well culture plates, after being induced by an osteogenic supplement (1×10-7mol/L dexamethasone, 5.0 mmol/L β-glycerophosphate, 50 mg/L ascorbic acid) and treated with daidzin, genistin, glycitin and 17β-estradiol at final concentrations of 1×10-8, 5×10-7, 1×10-6, 5×10-6, 1×10-5 mol/L for 7 d. The plates were washed thrice with ice-cold PBS and lysed by two cycles of freezing and thaw. Aliquots of supernatants were subjected to alkaline phosphatase activity and protein content measurement using an alkaline phosphatase kit and a micro-Bradford assay kit, respectively. The osteogenic differentiation rate was calculated according to the formula: ALP activitytreated/ALP activitycontrol×100%.
Oil red O stain and measurement The bone MSC and osteoblasts were isolated as above. MSC (3×106 cells per well) and osteoblasts (1×104 cells per well) were plated in 48-well culture plates, after being induced by adipogenic supplement (10 mg/L insulin, 1×10-7 mol/L dexamethasone and treated with daidzin, genistin, glycitin, and 17β-estradiol at final concentrations of 1×10-8, 5×10-7, 1×10-6, 5×10-6, 1×10-5 mol/L for 14 d and 9 d, respectively. Fat droplets within differentiated adipocytes from MSC and transdifferentiated adipocytes from osteoblasts were observed using the oil red O staining method described by Ichiro et al. with some modification[13]. Cell monolayers were fixed in 4% formaldehyde, washed in water and stained with a 0.6% (w/v) oil red O solution (60% isopropanol, 40% water) for 15 min at room temperature. For quantification, cell monolayers were then washed extensively with water to remove unbound dye, then 1 mL of isopropyl alcohol was added to the stained culture dish. After 5 min, the absorbance of the extract was assayed by a spectrophotometer at 510 nm. The adipogenic differentiation rate and adipocytic transdifferentiation rate were calculated according to the formula: ODtreated/ODcontrol ×100%.
Statistical analysis Data were expressed as mean±SD of at least three separate experiments. The statistical differences were analyzed using Student t-test.
Results
Effect of daidzin, genistin and glycitin on the MSC proliferation MTT tests showed that daidzin, genistin, and glycitin significantly promoted MSC proliferation and all the promotion rates were less than that of 17β-estradiol at the same concentrations (Figure 1).
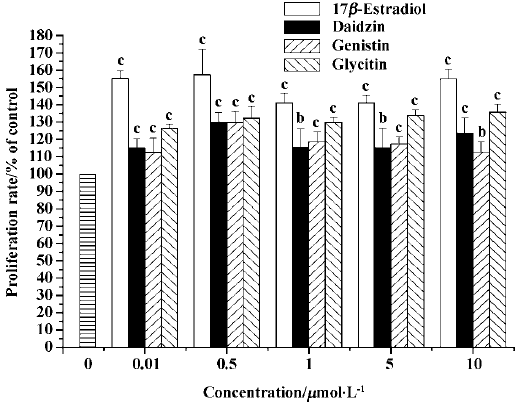
Effect of daidzin, genistin, and glycitin on osteoblast proliferation The daidzin, genistin, and glycitin significantly promoted osteoblast proliferation and the promotion rate of daidzin was greater than those of genistin and glycitin at the same concentrations (Figure 2).

Effect of daidzin, genistin, and glycitin on the osteogenic differentiation of MSC ALP activity measurements at d 7 showed that neither daidzin, genistin, and glycitin inhibited the osteogenic differentiation of MSC. Daidzin at concentration of 5×10-7mol/L and genistin at concentrations of 1×10-6, 5×10-6, and 1×10-5 mol/L promoted the osteogenic differentiation and all the promotion rates were less than those of 17β-estradiol at the same concentrations (Figure 3).

Effects of daidzin, genistin, and glycitin on the adipogenic differentiation of MSC Oil red O stain and measurement on d 14 showed that the effect of daidzin, genistin and glycitin on adipogenic differentiation depended on concentrations. Genistin inhibits adipogenic differentiation at concentrations of 1×10-8–1×10-5mol/L, and the effect was stronger than that of 17β-estradiol (Figure 4). The morphologic observation was in accordance with this result (Figure 5).


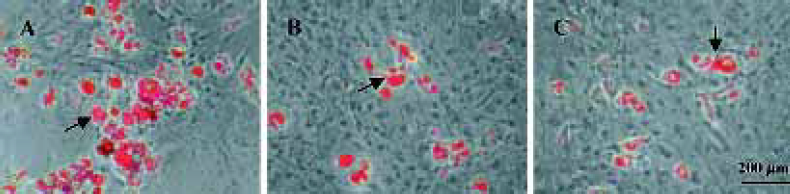
Effect of daidzin, genistin, and glycitin on adipocytic transdifferentiation of osteoblasts Oil red O stain and measurement showed that daidzin, genistin and glycitin inhibi-ted adipocytic transdifferentiation of osteoblasts at all concentrations in a dose-independent manner. The effects of these were stronger than that of 17β-estradiol (Figure 6). The morphologic observation was in accordance with the results (Figure 7).

Discussion
Adipocytic and osteogenic cells are believed to be derived from multipotential stromal cells in the marrow, and in vitro studies have shown an inverse relationship between the differentiation of adipocytic and osteogenic cells[5]. Besides having a passive role of space-filling in bone marrow cavity, recent data suggest that medullary adipocytes are secretory cells that may influence hematopoiesis and osteogenesis[14]. A variety of peptide and nonpeptide compounds are synthesized and released by adipocytes[15]. Adipocytes also secrete cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL-6), and the main effect of these cytokines is a stimulation of bone resorption[16]. In addition, Benayahu et al reported that preadipocytes had the potential to stimulate osteoclast differentiation[17]. On the relationship between adipogenesis and the ability to support osteoclast formation, Sakaguchi et al have demonstrated that adipocyte-enriched stromal cells also support osteoclast formation[18]. It has been reported that preadipo-cytes isolated from mouse marrow may regulate the activity and final differentiation of marrow precursors of osteoblasts. The condition medium harvested from mouse stromal preadipocytes decreased the alkaline phosphatase activity of a mouse stromal osteoblastic cell line[19]. So a reversal of adipogenesis will also be important as a therapeutic approach to treat age-related osteoporosis.
Osteonecrosis is a disabling condition that can be defined as the death of the cell components of the bone (including osteocytes and bone marrow cells). Possible pathogenesis of osteonecrosis is an increased size of marrow fat cells, a high intraosseous pressure, an accumulation of lipids within the osteocytes, and fat embolisms. Cui et al reported that steroid-induced adipogenesis by bone progenitor cells in the marrow might influence the development of osteonecrosis[20]. Lipid clearing agents, such as lovastatin, which promote osteogenic differentiation and inhibit adipogenic differentiation of MSC, might be helpful in preventing the development of steroid induced osteonecrosis.
In the present study we have examined the effects of daidzin, genistin, and glycitin on osteogenic and adipogenic differentiation of MSC and the adipogenic transdifferentiation of osteoblasts in an in vitro assay employing isolated mouse primary bone marrow stromal cells and osteoblasts. The results indicate that daidzin, genistin, and glycitin promoted osteogenic differentiation and inhibited adipogenic differentiation of MSC. It also inhibited adipocytic transdiffer-entiation of osteoblasts at appropriate concentrations similar to 17β-estradiol. These results were further supported by the fact that daidzin, genistin and glycitin did not inhibit proliferation of the MSC and osteoblasts. This suggests that daidzin, genistin and glycitin regulate a dual differentia-tional process of bone MSC into the osteogenic and adipogenic lineages, and transdifferentiational process of primary osteoblasts into the adipocyte lineages, causing a lineage shift toward osteoblasts. So, we deduced that the protective effects of daidzin, genistin, and glycitin on bone might be mediated by decreasing adipocytic cell formation from MSC. This may promote osteoblast proliferation, differentiation and mineralization, and secrete less cytokines, which may inhibit osteoclast formation and activation. In addition, the results also indicate that daidzin, genistin, and glycitin may be helpful in preventing the development of steroid induced osteonecrosis. The mechanisms of the effects of daidzin, genistin, and glycitin on the osteogenic and adipogenic differentiation of bone MSC, and the adipogenic transdifferentiation of osteoblasts, need to be studied further.
References
- Masayoshi Y. Isoflavone and bone metabolism: its cellular mechanism and preventive role in bone loss. J Health Sci 2002;48:209-22.
- Ahdjoudj S, Fromigue O, Marie PJ. Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: potential implication in the treatment of age-related bone loss. Histol Histopathol 2004;19:151-7.
- Dominici M, Hofmann TJ, Horwitz EM. Bone marrow mesenchymal cells: biological properties and clinical applications. J Biol Regul Homeost Agents 2001;15:28-37.
- Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARα2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone 2003;33:652-9.
- Beresford JN, Bennett JH, Devlin C, Leboy P, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 1992;102:341-51.
- Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 2000;27:177-84.
- Song CL, Dan GT. Adipocytes in marrow space and osteoporosis. Chin J Osteoporos 2002;8:266-9.
- Homma H, Kurachi H, Nishio Y, Takeda T, Yamamoto T, Adachi K, et al. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Bio Chem 2000;275:11404-11.
- Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, et al. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor(ER) α or β. Endocrinology 2002;143:2349-56.
- Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byer RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 2002;55:693-8.
- Thomas OC, Kathleen CM, Bruce E, Monica A, Thomas LM, Michael C, et al. Osteocalcin production in primary osteoblast cultures derived from normal and Hyp mice. Endocrinology 1998;139:35-43.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Katherine AK, Jeffrey MG. 1,25-Dihydroxy vitamin D3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology 1998;139:2622-8.
- Gimble JM. The function of adipocytes in the bone marrow stroma. New Biol 1990;2:304-12.
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr 1992;12:207-33.
- Mundy GR. Cytokines and bone remodeling. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego: Academic Press; 1996. p301.
- Benayahu D, Peled A, Zipori D. Myeloblastic cell line expresses osteoclastic properties following coculture with marrow stromal adipocytes. J Cell Biochem 1994;56:374-84.
- Sakaguchi K, Morita I, Murota S. Relationship between the ability to support differentiation of osteoclast-like cells and adipogenesis in murine stromal cells derived from bone marrow. Prostag Leukotr Ess 2000;62:319-27.
- Benayahu D, Zipori D, Wientroub S. Marrow adipocytes regulate growth and differentiation of osteoblasts. Biochem Biophys Res Commun 1993;197:1245-52.
- Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am 1997;79:1054-63.
