Honokiol inhibits arterial thrombosis through endothelial cell protection and stimulation of prostacyclin
Introduction
Platelet activation and aggregation play essential roles in thromboembolic disorders[1]. When platelets are activated they adhere to the injured blood vessel walls. This results in the formation of an occlusive thrombus in the lumen of the vessel[2,3]. These thrombi are the source of many thromboembolic cerebral vascular diseases, including strokes[4].
Honokiol is the main biphenyl neolignan isolated from Hou pu, the cortex of Magnolia officinalis (Magnoliaceae),
Materials and methods
Chemicals and reagents Honokiol injection was prepared by the Department of Natural Medicinal Chemistry, School of Pharmaceutical Sciences, Peking University (Beijing, China). This water-soluble preparation of honokiol contains polyvinyl and other auxiliary materials. Before use it was diluted with normal saline to different concentrations for intravenous injection (0.5 µg/mL, 5 µg/mL, 50 µg/mL for rats; 0.12 µg/mL, 1.2 µg/mL, 12 µg/mL for rabbits; concentrations vary with surface area of the animals). Acetylsalicylic acid (ASA) was the product of Astra (Wuxi, China) and was dissolved in normal saline by sonication before use. Collagen was purchased from Sigma (St Louis, MO, USA) and dissolved in normal saline before use. Oxidized low density lipoprotein (ox-LDL) was the product of Beijing Union Sanyou Science and Technology Development Co (Beijing, China). 6-keto-PGF1α [the stable metabolite of prostacyclin (PGI2)] immunoassay kits and nitric oxide (NO)/nitrate assay commercial kits were products of the Radioimmunology Institute of People Liberation Army General Hospital (Beijing, China). α-Tocopherol (VE) was the product of Beijing Double-Crane Pharmaceutical Co (Beijing, China). MTT was the product of Sigma. The control was normal saline plus defined proportion of auxiliary materials.
Experimental animals Male Sprague-Dawley rats and male New Zealand rabbits were obtained from the Experimental Animal Center of Peking University. The experimental procedures were approved by the Local Committee on Animal Care and Use.
Platelet aggregation study in vitro Blood samples were obtained from the auditive arteries of Male New Zealand rabbits weighing 3.0–4.0 kg into a syringe containing a 1:10 volume of 3.8% sodium citrate. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared by centrifugation of blood samples at 120×g and 850× g, respectively, for 15 min. The platelet count was adjusted to 1.8×109 platelets/mL with PPP. Platelet aggregation was measured using the 490 optical aggregometer (Chrono-log Co. Harvertown, PA USA) as described by Born[9]. Collagen was used as an inducer with a final concentration of 40–50 µg/mL.
Platelet aggregation study ex vivo Male rabbits weighing 3.0−3.5 kg were used after overnight fasting. The rabbits were administered intravenously with either control, honokiol 0.12, 1.2, and 12 µg/kg or ASA 1.2 mg/kg. Blood samples were then collected from the auditive arteries of the rabbits into a syringe containing a 1:10 volume of 3.8% sodium citrate at 10 min after administration. PRP and PPP were prepared as above. The platelet count was adjusted to 1.8×109 platelets/mL with PPP and platelet aggregation was measured with the Chrono-log mode 490 optical aggregometer. Collagen was used as an inducer with a final concentration of 40–50 µg/mL.
Carotid thrombosis model in rats induced by electric current Male Sprague-Dawley rats (280−320 g) were anesthetized with urethane (1.5 g/kg, ip) after overnight fasting. The left carotid arteries were then isolated carefully and treated intravenously with either control, honokiol 0.5, 5, 50 µg/kg or ASA 5 mg/kg. Carotid thrombus formation was induced using the modified Hladovec method[10] on an Electric Thrombosis Stimulator (BT87-3, Baotou Medical College, Baotou, China) by delivering a current of 3 mA for 3 min at 10 min after treatment. Occlusion time (OT) of the arteries was measured through a timer linked to the temperature sensor on the Thrombosis Stimulator.
Culture of rat aortic endothelial cells Endothelial cells were isolated from rat aorta by gentle scraping with vertical ophthalmic forceps. The rat aortic endothelial cells (RAEC) were then grown in T75 polystyrene flasks in the presence of antibiotics (100 U/mL penicillin G and 100 mg/mL streptomycin) and subcultured as described by Centra et al[11]. The endothelial cells were allowed to grow undisturbed for several days and thereafter the media were changed every 2 d for a total culturing period of 8–10 d. Cell culture purity (99%) was assessed by staining for factor VIII antigen, as described by Jaffe et al[12] and by visual inspection of their typical morphology. After mechanical disruption of cell monolayers by gente scraping and triturating, the cells were subcultured. Experiments were carried out using confluent cultures between passages 5 and 7.
Cultured rat aortic endothelial cell injury induced by ox-LDL Rat aortic endothelial cells of passage 5 were seeded at 1×105 cells/mL in 96-well plates and grown to confluence. The cells were treated with control or honokiol (final concentration 0.0376 µmol/L, 0.376 µmol/L or 3.76 µmol/L) or Vitamin E for 30 min in serum-free medium, and then incubated with ox-LDL (final concentration 50 µg/mL) for 24 h as described previously[13]. The viability of cells was assayed using the MTT assay.
Measurement of 6-keto-PGF1α levels in serum-free medium of injured rat aortic endothelial cells by ox-LDL The culture medium was removed after RAEC were grown to confluence, and cells were washed twice with phosphate-buffered saline and pre-incubated with the serum-free medium for 30 min. After the cells were treated with control, honokiol 0.376, 3.76, 37.6 µmol/L, or VE 100 mg/L at 37 oC for 30 min, the cultures were incubated with ox-LDL (final concentration 50 µg/mL) for 24 h to induce injury. Incubations were terminated by placing them in an ice bath. Culture fluids were saved for the determination of 6-keto-PGF1α, and cells were collected for protein content measurement. 6-keto-PGF1α was determined using immunoassay kits, and protein content was measured using the Bradford method[14].
Determination of nitric oxide levels in serum-free medium of injured rat aortic endothelial cells by ox-LDL Cells were cultured and treated as described above. The level of NO in culture fluid was determined using a kinetic cadmium-reduction method[15] with NO/nitrate assay commercial kits.
Statistical analysis The data were expressed as mean±SD and represent data from 5 repeated assays. Statistical evaluation was carried out using the Dunnet t-test to compare the differences between treated groups and control groups. P<0.05 was considered to be statistically significant.
Results
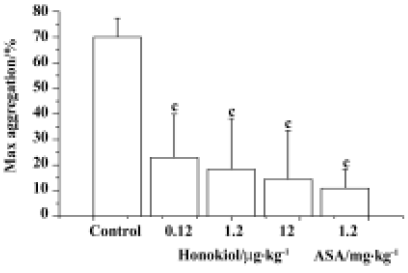
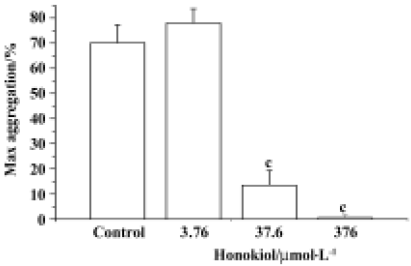
Effects of honokiol on rabbit platelet aggregation in vitro and ex vivo As shown in Figure 1, honokiol displayed a concentration-dependent inhibitory effect on platelet aggregation in vitro. Honokiol at 0.376 µmol/L did not influence platelet aggregation, but honokiol 37.6 µmol/L decreased the platelet aggregation from 78.0%±5.7% to 13.5%±5.9%, and an almost complete inhibition was observed when 376 µmol/L honokiol was used (from 78.0%±5.7% to 0.6%±1.1%). At 15 min after bolus intravenous administration, honokiol significantly inhibited platelet aggregation induced by collagen. The maximum aggregation rates were 70.0%±7.4% for the control group, 22.9%±17.0% for the 0.12 µg/kg group, 18.4%±19.8% for the 1.2 µg/kg group and 14.5%±19.0% for the 12 µg/kg group. It was evident that honokiol inhibited collagen-induced platelet aggregation in a dose-dependent manner. ASA, a typical anti-platelet agent, also inhibited collagen-induced platelet aggregation at 1.2 mg/kg under the same conditions (Figure 2). It is evident that honokiol has a potent inhibitory effect on collagen-induced platelet aggregation ex vivo; its effective dose is lower than that of ASA.
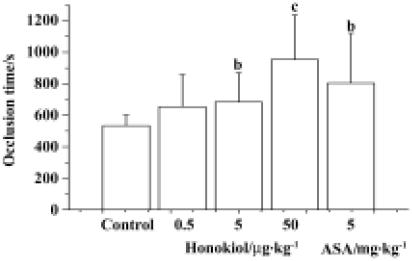
Effect of honokiol on electrical current-stimulated carotid thrombosis in rats The effects of honokiol on the carotid thrombus model in rats were measured at 10 min after bolus intravenous injection. The OT for the control group was 534 s±66 s, and for the 0.5 µg/kg, 5 µg/kg and 50 µg/kg of honokiol groups the OT were 654 s±204 s, 684 s±186 s and 954 s±282 s, respectively, while the OT for the 5 mg/kg ASA group was 804 s±318 s (Figure 3). It indicated that honokiol dose-dependently inhibited thrombosis induced by endothelium injury in vivo. ASA at 5 mg/kg also significantly inhibited thrombosis.
Effect of honokiol on viability of normal cultured rat aortic endothelial cells Honokiol 0.376–3.76 µmol/L significantly increased cell viability. However, honokiol 37.6 µmol/L and VE 100 mg/L did not influence cell viability. This suggested that low concentrations of honokiol could stimulate normal endothelial cell proliferation (Figure 4).
Protective effect of honokiol against cultured rat aortic endothelial cell injury induced by ox-LDL The ox-LDL-injured cells showed an obvious decrease in OD values compared with control-treated uninjured cells. Treatment of injured cells with honokiol 0.376–3.76 µmol/L significantly enhanced OD values in a concentration-dependent manner. Honokiol 37.6 µmol/L also increased OD value but no further increase was obtained compared with honokiol 3.76 µmol/L. It indicated that honokiol could protect endothelial cells against ox-LDL injury and increase cell viability. VE 100 mg/L also significantly increased cell viability (Figure 5).
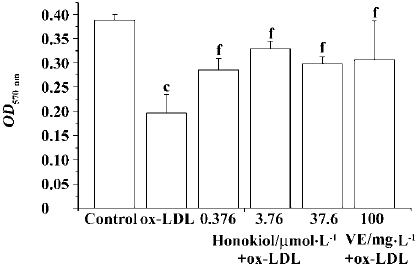
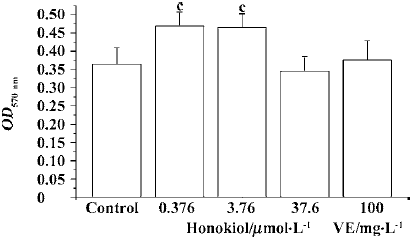
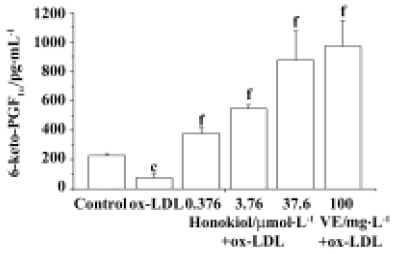
Influence of honokiol on 6-keto-PGF1α concentration in serum-free medium of rat aortic endothelial cells ox-LDL injury significantly decreased the PGI2 level in serum-free medium. Honokiol 0.376−36.7 µmol/L concentration-dependently increased 6-keto-PGF1α concentration in medium of injured rat aortic endothelial cells, which was significantly higher than that of injured cells and normal cells. VE 100 mg/L showed a similar result (Figure 6). These results demonstrated that both honokiol and VE were potent PGI2-release enhancers.
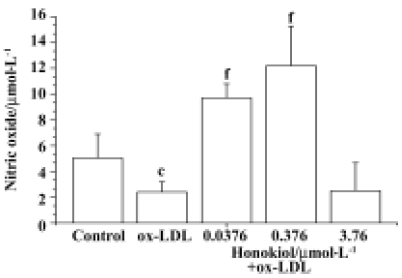
Effect of honokiol on nitric oxide concentration in serum-free medium of rat aortic endothelial cells Honokiol 0.0376–0.376 µmol/L significantly increased NO levels in serum-free medium of rat aortic endothelial cells. However honokiol 3.76 µmol/L did not increase NO level but slightly decreased its level. This result showed that appropriate concentrations of honokiol could promote NO release from rat aortic endothelial cells (Figure 7).
Discussion
Other authors have demonstrated that honokiol inhibits platelet aggregation in rabbits[5]. It has protective effects against myocardial ischemia[6,7] and cerebral infarction[8]. We have also found that honokiol can prevent cerebral injury caused by middle cerebral artery occlusion and cerebral ischemia reperfusion injury[16]. This study demonstrated that honokiol significantly inhibited arterial thrombosis induced by endothelium injury in rats, and the results on platelet aggregation in vivo and in vitro were basically consistent with those described previously[5]. We observed that the effective dose of honokiol was much lower than ASA, suggesting that honokiol is a potent platelet aggregation and thrombosis inhibitor. A previous study indicated that the anti-platelet aggregation activity of honokiol was due to its inhibitory effect on TXA2 formation and intracellular calcium mobilization[5]. In our preliminary study, honokiol significantly inhibited endothelium-injured thrombosis in rats, and its concentration for inhibiting platelet aggregation in vitro was 10 times higher than that for anti-thrombosis. We hypothesize that there is another mechanism mediating its anti-thrombotic effects.
Endothelial cell structure and functional integrity are important in the maintenance of blood vessel walls and circulatory function. They produce and release a variety of vasoactive substances, such as PGI2 and NO[17]. PGI2 is a potent endogenous platelet aggregation inhibitor and produces vasodilatation of all vascular beds studied[18,19]. We found that honokiol protected cultured RAEC from ox-LDL injury, and it potently increased PGI2 concentration in cell media in a concentration-dependant manner. The PGI2 level in cell media treated with honokiol and VE were much higher than that in normal cells, suggesting that both honokiol and VE not only protect cells from injury but also stimulate PGI2 release from normal RACE. This increase in PGI2 release may play a crucial role in the anti-thrombotic effect of honokiol. Simultaneously, we have observed that lower concentrations of honokiol 0.376–3.76 µmol/L stimulate normal RACE proliferation, and this effect may be important for maintaining vascular normal state and integrity as well as the concrescence of injured vessels. However, this effect of honokiol needs to be studied further.
At honokiol 37.6 µmol/L, the cell viability is not maximum but PGI2 level is highest. The reason for this phenomenon is not clear but it is indeed a fact that we confirmed with three separate experiments. We observed that cell shape had a little change at this concentration. Maybe honokiol at this concentration slightly inhibits cell growth or damages cell function. Under this condition the increase of PGI2 may be a response of cell to injurious stimulation[20]. This fact suggested that ideal effective concentration of honokiol should be less than 37.6 µmol/L.
According to Teng et al, honokiol inhibited TXA2 formation in platelets[5]. This may be due to inhibition of enzymes involved in TXA2 synthesis, including cyclooxygenase (COX) and TXA2 synthase in platelets. But our findings suggested that honokiol stimulated PGI2 release from endothelial cells. A possible explanation for this is that honokiol inhibits TXA2 synthase in platelets but activates PGI2 synthase in endothelial cells. However, definitive evidence needs to be provided.
The continuous release of NO from the endothelium has an important role in blood flow modulation. NO may also modulate interactions between inflammatory cells and the endothelium. It reduces platelet, monocyte, macrophages, and neutrophils adhesion to endothelial cells and inhibits platelet aggregation[21–25]. This study showed that honokiol 0.0376−0.376 µmol/L stimulated NO release, but honokiol 3.76 µmol/L did not and caused a slight decrease in NO level. The effective concentration range of honokiol in stimulating NO release is 10 times lower than that in increasing cell viability. Thereby NO release may not play an important role in the anti-thrombotic effect of honokiol. Accordingly, protection of endothelial cells and stimulation of PGI2 release from endothelial cells may be the main mechanism by which honokiol inhibited thrombosis, apart from its inhibitory effect on platelet arachidonic acid pathway as described by Teng et al[5]. However, honokiol and VE showed similar effects in cell protection and PGI2 release under present condition. Whether these effects are the results of their anti-oxidative properties or they themselves have a stimulatory effect on PGI2 release is not clear yet. In addition, the results of this study cannot explain whether honokiol stimulates PGI2 generation in endothelial cells. The limitation of this study is that it did not supply direct evidence of the importance of PGI2 on anti-thrombosis caused by honokiol.
In conclusion, this study showed that honokiol is a potent arterial thrombosis inhibitor. Protection of endothelial cells and stimulation of PGI2 release may be the main mechanism. NO release from endothelial cells may play some roles but is not a key factor. The detailed mechanism by which honokiol stimulates PGI2 release and promotes endo-thelial cell proliferation requires further study.
References
- Fitzgerald DJ, Roy L, Catella F, Fitzgerald GA. Platelet activation in unstable coronary disease. New Engl J Med 1986;315:983-9.
- Fuster V, Chesebro JH. Mechanisms of unstable angina. New Engl J Med 1986;315:1023-5.
- Willerson JT, Hillis LD, Winniford M, Buja LM. Speculation regarding mechanisms responsible for acute ischemic heart disease syndromes. J Am Coll Cardiol 1986;8:245-51.
- Pachham MA. Role of platelets in thrombosis and homeostasis. Can J Physiol Pharmacol 1994;72:278-84.
- Teng CM, Chen CC, Ko FN, Lee LG, Huang TF, Chen YP, et al. Two anti-platelet agents from Magnolia officinalis. Thromb Res 1988;50:757-65.
- Tsai SK, Huang SS, Hong CY. Myocardial protective effect of honokiol: an active component in Magolia officinalis. Planta Med 1996;62:503-06.
- Tsai SK, Huang CH, Huang SS, Hung LM, Hong CY. Antiarrhythmic effect of magnolol and honokiol during acute phase of coronary occlusion in anesthetized rats: influence of L-NAME and aspirin. Pharmacology 1999;59:227-33.
- Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia–reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res 2003;992:159-66.
- Born GVR. Aggregation of blood platelet by adenosine diphosphate and its reversal. Nature 1962;194:927-9.
- Hladovec J. Experimental arterial thrombosis in rats with continuous registration. Thromb Diath Haemorrh 1974;26:407-10.
- Centra M, Ratych RE, Cao GL, Li J, Williams E, Taylor RM, et al. Culture of bovine pulmonary artery endothelial cells on Gelfoam blocks. FASEB J 1992;6:3117-21.
- Jaffe EA, Hoyer LW, Nachman RL. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest 1973;52:2757-64.
- Yan JC, Liu NF, Chen RX. Effects of oxidized low density lipoprotein and vitamin E on nitric oxide production and nitric oxide synthase activity in cultured human umbilical vein endothelial cells. Chin J Arterioscler 1999;7:145-8.
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dyebinding. Anal Biochem 1976;72:248-54.
- Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 1990;36:1440-4.
- Chen SZ, Wang H, Wang YY, Hu YH, inventors; Beijing Kelong Huanyu Patent Office, assignee. Application of honokiol, magnolol and their preparation in cardiovascular and cerebral vascular diseases. Chinese patent: 200310121303.0. 2005 Jun 15.
- Sumpio BE, Riley JT, Dardik A. Molecules in focus, cells in focus: endothelial cell. Int J Biochem Cell Biol 2002;34:1508-12.
- Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci USA 1977;74:3922-7.
- Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides thromboxane A2 and prostacyclin. Pharmacol Rev 1978;30:293-31.
- Miceli F, Tringali G, Tropea A, Minici F, Orlando MT, Lanzonec A, et al. The effects of nitric oxide on prostanoid production and release by human umbilical vein endothelial cells. Life Sci 2003;73:2533-42.
- Bath PM, Hassall DG, Gladwin AM, Palmer RM, Martin JF. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler Thromb 1991;11:254-60.
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 1991;88:4651-5.
- Radomski MW, Vallance P, Whitley G, Foxwell N, Moncada S. Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc Res 1993;27:1380-2.
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Raja-vashisth TB, Gimbrone MA, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflamma-tory cytokines. J Clin Invest 1995;96:60-8.
- Cartwright JE, Johnstone AP, Whitley GJ. Inhibition of nitric oxide synthase by antisense techniques: investigations of the roles of NO produced by murine macrophages. Br J Pharmacol 1997;120:146-52.
