Multiple dose pharmacokinetics of risperidone and 9-hydroxyrisperidone in Chinese female patients with schizophrenia
Introduction
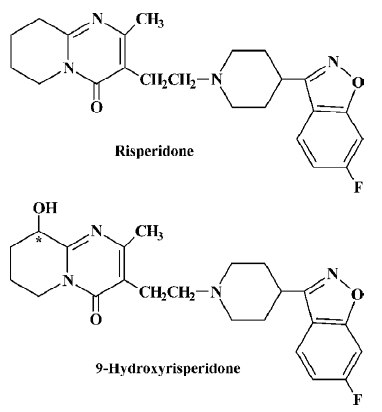
Risperidone (RIS; Figure 1), a benzisoxazole derivative, is a relatively new antipsychotic agent that combines serotonin type 2 (5HT2) and dopamine type 2 (D2) receptor antagonism[1]. RIS has been shown to be an effective antipsychotic, affecting both the positive and negative symptoms of schizophrenia, with a low incidence of extrapyramidal symptoms (EPS) and a lack of anticholinergic effects[2–3]. When administered orally to healthy volunteers, RIS was absorbed rapidly, achieved a peak plasma concentration within 2.14 h[4], and displayed linear pharmacokinetics at doses between 0.5 mg and 25 mg[5]. RIS is extensively metabolized in the liver by CYP2D6 to a major metabolite, 9-hydroxyrisperidone (9-OH-RIS; Figure 1)[6–7], which is eliminated by renal excretion[8], and CYP3A4 also catalyzes the formation of (-)-9-hydroxyrisperidone in vitro[9–10]. Studies in animals[8] and receptor binding in vitro[8] and in vivo[11] indicate that 9-OH-RIS has approximately 70% of the pharmacological activity of RIS. Therefore, the total active risperidone moiety (RIS plus 9-OH-RIS) is considered to be the most clinically relevant measure[12]. Although RIS has a half-life of 2–4 h in CYP2D6 extensive metabolizers and up to 20 h in poor metabolizers, the pharmacokinetic profile of the active moiety after single and multiple doses was similar in extensive and poor metabolizers, with an overall mean elimination half-life of 20 h[6,12–14].
Risperidone has been widely used to treat schizophrenia or schizophreniform disorders after it was put on the market in 1993 in China. However, the steady-state pharmacokinetics of RIS in Chinese patients, especially Chinese female patients, who, relative to Chinese male patients, have a higher prevalence of schizophrenia[15–20], higher serum levels of prolactin after administration of RIS[21], receive less family concern, and have lower educational levels[22,23], has not previously been systematically studied. The aim of the present study was to determine the pharmacokinetics of repeated oral doses and to document the safety of RIS in Chinese female patients suffering from schizophrenia or schizophreni-form diseases.
Materials and methods
Drugs and reagents RIS (purity 99%) and 9-OH-RIS (purity 99%) standards were donated by Xi’an Janssen Pharmaceutical (Xi’an, Shanxi, China). Quetiapine (IS; purity >99.6%) was kindly provided by Hunan Dongting Pharmaceutical (Changde, Hunan, China). RIS tablets (batch no: AM 010910767, 1 mg/tablet) were kindly donated by Xi’an Janssen Pharmaceutical. High performance liquid chromatography (HPLC) grade reagents (methanol, acetonitrile and ammonium acetate) were purchased from Tedia (Fairfield, OH, USA). Other AR grade reagents were purchased from the Chemical Reagents Factory of Hunan province (Changsha, Hunan, China).
Apparatus Waters 2690 HPLC equipment system, micromass ZQ mass spectrometer (Wythenshawe, Manchester, UK) equipped with an electrospray ionization (ESI) ion source, Compaq Deskpro Workstation, and Masslynx 3.5 software were used.
Study subjects Chinese female in-patients aged 18–65 years were recruited to the study. All the subjects were diagnosed with schizophrenia or schizophreniform disorders according to the Chinese Criteria of Mental Disorders (CCMD, 3rd edition), completed during 1996–2000 by 114 renowned Chinese psychiatrists with reference to ICD-10 from WHO and DSM-IV from American. On the basis of their medical history, a physical examination and routine laboratory tests, no patients had hepatic, renal, cardiac, hematological or other diseases. Cigarettes and alcohol were restrained. Patients who were also being treated with drugs known to inhibit or induce the activity of CYP2D6 or CYP3A4 were excluded. Written informed consent was obtained from each parent or patients’ legal guardian. The Ethical Committee of Xiangya Second Hospital of Central South University approved the protocol.
Experimental protocol The present study was an open label and single-center trial. All the subjects were treated using a titration scheme. The titration scheme comprised 0.5 mg doses of RIS twice daily (bid) for 2 d, 1 mg bid for 5 d, 2 mg bid for 7 d, followed by 2 mg qd for 1 d. On d 15, following an overnight fast, a final dose of RIS was administered in the morning and serial blood samples (2–3 mL) were collected before the final dose and at 0.5, 1.25, 2, 3, 4, 6, 8, 12, 24, and 48 h after the final dose. To confirm the steady-state concentrations of RIS and 9-OH-RIS, blood samples for trough plasma concentrations were collected before the morning dose of RIS on d 13 and 14. Plasma was separated by centrifugation and stored at -80 ºC. During the trial, all subjects had the same diet.
Safety assessments Adverse events (AE) were monitored throughout the trial, together with an assessment of their severity and possible relationship to the administration of RIS. A complete physical examination was performed at screening and at the end of the study. Vital signs, including blood pressure and pulse rate, were measured at screening, d 0 and 16, and at the end of the study. A standardized 12-lead electrocardiogram (ECG) was performed at screening and on d 0 and 16. Clinical laboratory tests, including hematology and clinical chemistry, were performed at screening and on d 0 and 16. Serum prolactin concentration was determined at 7:00 AM on d 0 and 16, and at the end of the study.
Analytical methods Plasma concentrations of RIS and 9-OH-RIS were determined using a validated procedure described elsewhere[24,25], involving liquid-liquid extraction of RIS and 9-OH-RIS and detection by high-performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-MS). Calibration curves were linear over the concentration range of 0.5 to 200 µg/L for RIS and 5 to 250 µg/L for 9-hydroxyrisperidone. The correlation coefficient obtained by linear regression were 0.9905 for RIS, and 0.9926 for 9-OH-RIS. The limit of quantification was 0.5 µg/L for RIS and 5 µg/L for 9-OH-RIS. Recovery of RIS and 9-OH-RIS was examined at 3 different concentrations. The recovery rates were greater than 80.3% for RIS, and greater than 78.4% for 9-OH-RIS. Intra- and interassay variability was examined at 3 concentrations for RIS and 9-OH-RIS. The intra-assay variabilities, expressed as coefficients of variation (n=5) were less than 8.1% for RIS, and less than 10.1% for 9-OH-RIS. The interassay variabilities were less than 12.8% for RIS and less than 14.2% for 9-OH-RIS.
Pharmacokinetic analysis The steady-state peak concentration (Cssmax) and the time to the peak concentration (Tmax) were recorded as observed. The terminal elimination rate constant (Ke) was determined by linear regression of the terminal points of the log-linear plasma concentration-time curve. The terminal-phase elimination half-life (T1/2) was calculated as 0.693/Ke. AUCss0–12 was calculated by using the linear trapezoidal rule. The apparent volume of clearance (CL/F) and distribution (V/F) of RIS were calculated as dose/AUCss0–12 and CL/Ke, respectively. The steady-state trough plasma concentration (Cssmix) was represented by the plasma concentration collected before RIS administration on d 18. The steady-state average plasma concentration for the 0–12 h dosing interval (Cssav) was calculated as AUCss0–12/12. The pharmacokinetic parameters of the active moiety (RIS plus 9-OH-RIS) were also calculated.
Statistical analysis All values are expressed as mean±SE (range). All statistical tests were 2-tailed and significance was set at the 0.05 level. Differences in the mean values of physical examinations and clinical laboratory tests before and after the study were compared by using a paired t-test.
Results
Forty subjects were originally enrolled, but 17 of these were excluded because they were outpatients or were comedicated with drugs that might have interfered with the pharmacokinetics of the study drugs or interpretation of the results. The most frequent comedications were benzodiaze-pines, antiparkinsonian drugs, antiepileptics, analgesics, and cardiovascular drugs. A total of 23 subjects (age 28.3±9.1; BMI 23.0±3.1) completed the present pharmacokinetics study for RIS. All the subjects were identified as extensive or medium metabolizers of CYP2D6 by phenotyping (dextrometh-morphan was used as the probe drug).
The trough plasma concentrations of RIS and its metabolite, 9-OH-RIS, on d 13, 14, and 15 were not significantly different (P>0.05), indicating that steady-state concentrations of RIS and its metabolite were achieved.
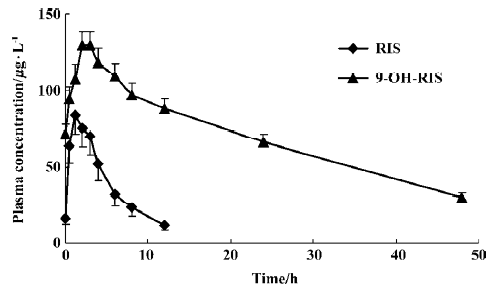
The mean pharmacokinetic parameters of RIS, 9-OH-RIS, and the active moiety (RIS plus 9-OH-RIS) are summarized in Table 1. Mean steady-state plasma concentration-time curves are shown in Figure 2. After oral administration, RIS was rapidly absorbed with a mean Tmax of 1.6 h and 9-OH-RIS was quickly metabolized from the parent drug with a mean Tmax of 2.5 h. Pharmacokinetic studies indicated that RIS had a mean half-life of 3.2 h, a small volume of distribution (approximately 34.1 L), and a low clearance (approximately 8.7 L/h); 9-OH-RIS had a long mean half-life of 24.7 h.
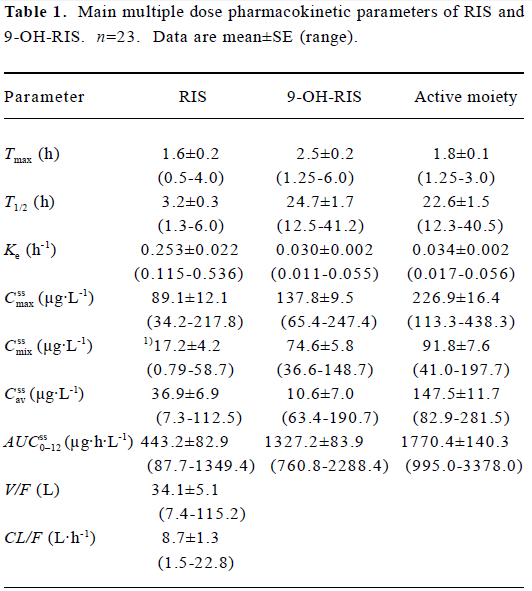
Full table
We found that the pharmacokinetics parameters of RIS varied greatly among individuals. Among the 23 patients, there were 4 patients (approximately 17%) whose Cssmax of RIS was below the limit of detection (0.5 µg/L). The coefficients of variation (CV) of V/F and CL/F were all 71% for RIS. The CV of Cssmix was 105% for RIS. AUCss0–12 also had a large CV, and the CV of RIS was 90%. For T1/2, the CV of RIS was 40%. Compared with that of RIS, the pharmacokinetic parameters of 9-OH-RIS had lower CV values (Cssmix 37%; AUCss0–12 30%; T1/2 32%). For the active moiety, the CV values of Cssmix, AUCss0–12, and T1/2 were 40%, 38%, and 32%, respectively.
Table 2 summarizes the overall incidence of AE according to the preferred terms. No deaths or serious side effects were reported during the study. No subject developed extrapyramidal side effects following the administration of RIS. No clinically significant abnormal physical examination findings, ECG results, laboratory values or vital signs was observed during the study. However, serum prolactin chang-ed significantly (P<0.05), increasing from 14.1±7.6 µg/L before RIS was administered, to 87.2±35.7 µg/L after administration of RIS. There was no correlation between serum prolactin concentration and the concentration of RIS, 9-OH-RIS, or the active moiety. Twenty-three patients experienced a total of 35 AE, of which the majority were rated mild in intensity. In patients receiving RIS, 30 of 35 AE were considered possibly or probably related to the administration of RIS.
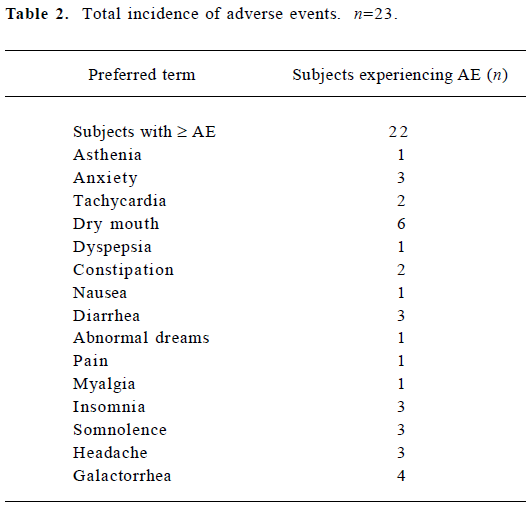
Full table
Discussion
When compared with data obtained in a population of white people with schizophrenia[26], our data show that Chinese female patients suffering from schizophrenia have higher steady-state trough plasma concentrations (Cssmix) of RIS and 9-OH-RIS. In our study the of RIS and 9-OH-RIS were 19.3 and 79.5 µg/L, respectively; however, the corresponding values were found to be 2.9 and 24.1 µg/L, respectively, in the previous study[26]. A similar study also indicated that plasma levels of the antipsychotic and its metabolite are at least 2–3 times higher in Chinese female subjects than in their Western counterparts[27]. After dose-adjusting the of RIS and 9-OH-RIS in our study (RIS 221.6±198.7 µg·h·L-1; 9-OH-RIS 663.6±201.2 µg·h·L-1), which were much higher than corresponding values found in two previous studies (RIS 41.6±23.4 µg·h·L-1; 9-OH-RIS 193.4±76.5 µg·h·L-1[28])(RIS 59.6±16.3 µg·h·L-1; 9-OH-RIS 162.1±19.2 µg·h·L-1[29]). Some well-known inter-ethnic differences in drug metabolism deserve to be considered. There are some ethnic characteristics that might have contributed to this finding: for example the activity of the metabolic enzymes (CYP2D6 or CYP3A4), body weight, and lean body mass. Additional investigations are needed to explain this observation. In any case, the discovery of the relatively higher RIS and 9-OH-RIS plasma concentrations in Chinese female patients may be useful in optimizing the clinical treatment protocol for RIS.
The present study shows that the pharmacokinetic parameters of RIS show large interindividual variability in Chinese female patients. This is consistent with the results of a previous study[26,30]. Variability between patients was notable for RIS, but remained modest for 9-OH-RIS in our study. The reason for this might be that 9-OH-RIS is eliminated by renal excretion, whereas RIS is extensively metabolized by the enzyme CYP2D6, which has high intersubject variability in intrinsic metabolic capacity, despite the fact that there is a much lower incidence of poor metabolizers in the Asian population[31–36]. When the clinically relevant psychoactive moiety, consisting of the sum of RIS plus 9-OH-RIS, was measured, the CV of the pharmacokinetic parameters was lower than that of RIS; ie, there was a reduction in the profound differences in plasma concentrations between individuals.
The absolute bioavailability of RIS is approximately 70%[5,12], which clearly indicates that there is a first-pass effect for RIS. Of note, an earlier study demonstrated that RIS is a substrate of the P-glycoprotein (P-gp), a kind of transmembrane transporter of an ATP-dependent efflux pump for a wide range of drugs[37]. In the human gastrointestinal tract, P-gp is found in high concentrations on the apical surfaces of superficial columnar epithelial cells of the colon and distal small bowel. High levels of P-gp are also found on the apical surfaces of epithelial cells in the small biliary ductules, small ductules of the pancreas, proximal ductules of the kidneys, and adrenal glands. P-gp is richly expressed on the subapical surface of the epithelium of the choroids plexus of the brain (which forms the blood-cerebrospinal fluid barrier) as well as the luminal surface of the endothelium of the blood capillaries of the brain (blood-brain barrier)[38–41]. P-gp functions to limit the absorption and, potentially, systemic exposure to its substrates (eg risperidone, cyclosporine, tacrolimus, and talinolol). Intestinal P-gp also exhibits wide interindividual variation in its expression (8- to 10-fold)[42]. Whether the metabolizing enzymes (CYP2D6 or CYP3A4) or P-gp, or both primarily contribute to interindividual variability is a topic for further study.
RIS treatment was conducted safely in all 23 subjects. However, in our study RIS treatment resulted in high serum prolactin, which was consistent with previous studies[43,44]. Knegtering et al indicated that the plasma concentration of 9-OH-RIS correlated significantly with increases in plasma prolactin[44]. A recent study also showed that plasma concentrations of the RIS active moiety might play a part in predicting the clinical response and occurrence of extrapyramidal symptoms when treating patients with RIS[45]. Therefore, routine therapeutic drug monitoring may be useful to optimize the treatment protocol. For Chinese female patients, an additional investigation with more samples is needed to acquire clearer results.
In conclusion, RIS showed large interindividual variations in pharmacokinetic parameters, indicating that systemic exposure to RIS and 9-OH-RIS in female Chinese schizophrenic patients is higher than that experienced by white Caucasian patients. Doses for individual patients should be carefully titrated and the patients' prolactin levels should be monitored carefully, to minimize side effects. Larger studies regarding the PK/PD relationship might be needed to determine the optimal dose of RIS in Chinese female patients.
Acknowledgement
The authors thank Xi'an Janssen Pharmaceutical (Xi'an, China) for donating the risperidone tablets and all nurses in the women’s ward of the Xiangya Second Hospital Psychiatry Department for their enthusiastic clinical assistance.
References
- Janssen PA, Niemegeers CJ, Awouters F, Schellekens KH, Megens AA, Meert TF. Pharmacology of risperidone (R64766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. J Pharmacol Exp Ther 1988;244:685-93.
- Marder SR, Davis DM, Chouinard D. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997;58:538-46.
- Mesotten F, Suy E, Pietquin M, Burton P, Heylen S, Gelders Y. Therapeutic effect and safety of increasing doses of risperidone (R64766) in psychotic patients. Psychopharmacology 1989;99:445-9.
- Huang F, Lasseter KC, Janssens L. Pharmacokinetics and safety assessments of Galantamine and Risperidone after the two drugs are administered alone and together. J Clin Pharmacol 2002;42:1341-51.
- He H, Richardson JS. A pharmacological, pharmacokinetic and clinical overview of risperidone, a new antipsychotic that blocks serotonin 5-HT2 and dopamine D2 receptors. Int Clin Psycho-pharmacol 1995;10:19-30.
- Mannens G, Huang ML, Meuldermans W. Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos 1993;21:1134-41.
- Koymans L, Vermeulen NP, van Acker SA. A predictive model for substrates of cytochrome P450-debrisoquine (2D6). Chem Res Toxicol 1992;5:211-9.
- Beijsterveldt V, Geerts JFR, Lseysen JE. The regional brain distribution of risperidone and its active metabolite 9-hydroxy-risperidone in the rat. Psychopharmacol 1993;15:371-9.
- Fang J, Bourin M, Baker GB. Metabolism of risperidone to 9-hydroxyrisperidone by human cytochromes P450 2D6 and 3A4. Naunyn Schmiedebergs Arch Pharmacol 1999;359:147-51.
- Yasui-Furukori N, Hidestrand M, Spina E. Different enantioselective 9-hydroxylation of risperidone by the two human CYP2D6 and CYP3A4 enzymes. Drug Metab Dispos 2001;29:1263-8.
- Nyberg S, Dahl ML, Halldin C. A PET study of D2 and 5-HT2 receptor occupancy induced by risperidone in poor metabolizers of debrisoquin and risperidone. Psychopharmacology (Berl) 1995;119:345-8.
- Huang ML, van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, et al. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther 1993;54:257-68.
- Heykants J, Huang ML, Mannens G, Meuldermans W, Snoeck E, Van Beijsterveldt L, et al. The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatry 1994;55 Suppl:13-7.
- Ereshefsky L, Lacombe S. Pharmacological profile of risperidone. Can J Psychiatry 1993;38 Suppl 3:S80-8.
- Wei X. To review the data of epidemiological survey of schizophrenia. Chin Gen Practice 2003;8:669-70.
- Shen YC. Discussion of the data of prevalence survey of all kinds of mental diseases. Chin J Neurol Psychiatry 1986;19:80-2.
- Shen YC, Chen CH. Analysis of the data and methodology of epidemiological survey of schizophrenia in 12 Chinese regions. Chin J Neurol Psychiatry 1986;19:65-9.
- Xi W. An epidemiological survey of schizophrenia inpatients. Chin J Behav Med Sci 1999;34:50-1.
- Wang C. A prevalence survey of schizophrenia in Yichun city of Jiang Xi Province. Sichuan Psychiatry Sci 2002;15:178-9.
- Wang SJ. An epidemiological survey on schizophrenia in Fuyang, Anhui (1984-1994). J Chin Psychol Med 2002;12:3-4.
- Cui LJ, Tian GQ, Zhao SM. Serum levels of prolactin and growth hormone and effect of pharmacotherapy on schizophrenia: a comparative study of risperidone and chlorpromazine. Chin Mental Health J 2000;13:368-70.
- Gao ZS, Wu SY. Sex differences of social psychic factors effecting on community patients with schizophrenia. J Clin Psychosomatic Diseases 1999;3:1-3.
- Liu GQ, Xiang MZ, Yang MS. Study of the sex differences of patients with schizophrenia in the community of countryside. J North Sichuan Med College 1998;13:70-1.
- Zhou Z, Li X, Li K, Li H. Simultaneous determination of clozapine, olanzapine, risperidone and quetiapine in plasma by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromat B 2004;802:257-62.
- Li KY, Cheng ZN, Li X, Bai XL, Zhang BK, Wang F, et al. Simultaneous determination of quetiapine and three metabolites in human plasma by high-performance liquid chromatography-electrospray ionization mass spectrometry. Acta Pharmacol Sin 2004;25:110-4.
- Balant-Gorgia AE, Androniki E. Therapeutic drug monitoring of risperidone using a new, rapid HPLC method: Reappraisal of interindividual variability factors. Ther Drug Monit 1999;21:105-15.
- Spina E, Avenoso A, Facciola G. Relationship between plasma risperidone and 9-hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology (Berl) 2001;153:238-43.
- Bondolfi G, Eap CB, Bertschy G. The effect of fluoxetine on the pharmacokinetics and safety of risperidone in psychiatric patients. Pharmacopsychiatry 2002;35:50-6.
- Reyes JF, Preskorn SH, Khan A. Concurrent administration of donepezil HCl and risperidone in patients with schizophrenia: assessment of pharmacokinetic changes and safety following multiple oral doses. Br J Clin Pharmacol 2004;58 Suppl 1:50-7.
- Castberg I, Spigset O. Serum concentrations of risperidone and 9-hydroxyrisperidone after administration of the long-acting injectable form of risperidone: evidence from a routine therapeutic drug monitoring service. Ther Drug Monit 2005;27:103-6.
- Transon C, Lecoeur S, Leemann T, Beaune P, Dayer P. Interindividual variability in catalytic activity and immunoreactivity of three major human liver cytochrome P450 isozymes. Eur J Clin Pharmacol 1996;51:79-85.
- Shu Y, Cheng ZN, Liu ZQ, Wang LS, Zhu B, Huang SL, et al. Interindividual variations in levels and activities of cytochrome P-450 in liver microsomes of Chinese subjects. Acta Pharmacol Sin 2001;22:283-8.
- Berecz R, Dorado P, De La Rubia A, Caceres MC, Degrell I, Lerena A. The role of cytochrome P450 enzymes in the metabolism of risperidone and its clinical relevance for drug interactions. Curr Drug Targets 2004;5:573-9.
- Han XM, Zhou HH. Polymorphism of CYP450 and cancer susceptibility. Acta Pharmacol Sin 2000;21:673-9.
- Chen SQ, Wedlund PJ. Correlation between cytochrome P-450 CYP2D6 (CYP2D6) genotype and phenotype. Acta Pharmacol Sin 1999;20:585-8.
- Cai WM, Chen B, Liu YX, Chu X. Dextromethorphan metabolic phenotyping in a Chinese population. Acta Pharmacol Sin 1997;18:441-4.
- Boulton DW, DeVane CL, Liston HL. In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci 2002;71:163-9.
- Thiebaut F, Tsuruo T, Hamada H. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 1987;84:7735-8.
- Yang HT, Wang GJ. Transport and uptake characteristics of a new derivative of berberine (CPU-86017) by human intestinal epithelial cell line: Caco-2. Acta Pharmacol Sin 2003;24:1185-91.
- Rao VV, Dahlheimer JL, Bardgett ME. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood- cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA 1999;96:3900-5.
- He L, Liu GQ. Interaction of multidrug resistance reversal agents with P-glycoprotein ATPase activity on blood-brain barrier. Acta Pharmacol Sin 2002;23:423-9.
- Ayrton A, Morgan P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 2001;31:469-97.
- Deberdt WG, Dysken MW, Rappaport SA. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry 2005;13:722-30.
- Knegtering R, Baselmans P, Castelein S. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry 2005;162:1010-2.
- Kakihara S, Yoshimura R, Shinkai K, Matsumoto C, Goto M, Kaji K, et al. Prediction of response to risperidone treatment with respect to plasma concentrations of risperidone, catecholamine metabolites, and polymorphism of cytochrome P450 2D6. Int Clin Psychopharmacol 2005;20:71-8.