Involvement of GABA and opioid peptide receptors in sevoflurane-induced antinociception in rat spinal cord
Introduction
Gamma-aminobutyric acid (GABA) and opioid peptides are the major inhibitory transmitters in the central nervous system, and both transmitter systems are likely to play important roles in anesthetic mechanisms. For example, a common property of a wide spectrum of general anesthetics, including barbiturates and propofol, is enhancement of the action of GABAA receptors[1–4].
Increasing evidence have indicated that the spinal cord is pivotal in the immobility induced by volatile anesthetics because the anesthetics depress the activity of α-motoneurons in the spinal cord[5,6], in which process spinal GABA receptors and opioid peptide receptors may be involved[7–10]. However, there has been few study on the effects of sevoflurane on pain processing in the spinal cord. Given the high density of GABA receptors and opioid peptide receptors located in the dorsal horn of the spinal cord, in the present study we examine whether the suppression of nociceptive effects by sevoflurane is mediated by actions at GABAA and opioid receptors in the spinal cord.
Materials and methods
Animals and surgical preparation This study was approved by animal experimentation committee of Xinhua hospital. The experiments were performed on 37 male Sprague-Dawley rats weighing 215–345 g (provided by the Animal Center of the Chinese Academy of Sciences). Animals were divided into 5 groups according to the different tests. Group I (n=6) animals were used to test the effects of sevoflurane after transection of the spinal cord. Anesthesia for surgical preparation was induced by 2.5% sevoflurane in 95% O2–5% N2 and maintained by 0.8%–1.5% sevoflurane through a tightly fitting mask. Group II (n=11) animals were used to test the time course of sevoflurane in the spinally transected rats; Group III (n=9), group IV (n=6) and group V (n=5) animals were used to test the effect of bicuculline (GABAA receptor antagonist), phaclofen (GABAB receptor antagonist) and naloxone (opioid receptor antagonist), respectively. Animals in groups II–V were anesthetized with urethane (1.0 g/kg, ip). The trachea, left internal jugular vein and carotid artery were cannulated for artificial ventilation, drug administration and blood pressure monitoring, respectively.
Following laminectomy, the spinal cord was transected at T9–T10 with 2.0% lidocaine surface anesthesia. The experiments were started at least 2 h after transection. During experiments, core body temperature was maintained at 37–39 ºC by a warm blanket and a heat lamp. In order to maintain effective circulation, 0.5–1 mL 5% glucose was injected into the internal jugular vein every 1–2 h.
Electrophysiological recording Electromyographic measurements were taken in the gastrocnemius muscles. Two stainless steel needles were separately inserted into the two toes of the ipsilateral hindpaw for electrical stimulation. The intensity of electrical stimulation (1 ms, 50 V, 3 pulses, 1 Hz) was sufficient to excite the C-fiber nociceptors. Two components of the electromyographic response, the A- and C-responses, were evoked by the electrical stimulation. The C response was an indication of nociceptive reflex. An averaged C response was analyzed by a signal processing system (SMUP-PC, Fudan University).
Drug administration In group I, sevoflurane was delivered at concentrations of 0.8%, 1.0%, 1.5%, 2.0%, and 2.5%. Electromyographic measurements were taken 25 min after inhalation of sevoflurane. In group II, 1.0% sevoflurane was given, and electromyographic measurements were taken every 3 min. In groups III, IV, and V, as a control, bicuculline (0.1 mg/kg), phaclofen (0.6 mg/kg) or naloxone (0.4 mg/kg) were first intravenously administered without inhalation of sevoflurane. Two hours later, bicuculline, phaclofen, or naloxone at the same doses was administered, respectively, 15 min after inhalation of 1.0% sevoflurane.
Data analysis The effects of drugs, dose dependence, and time course were analyzed by using the paired t-test. Differences between groups were determined to be statistically significant if P<0.05. All data are shown as mean±SD.
Results
Consistent with our previous study[11], the strong current-induced electromyographic responses from the gastrocnemius muscle consisted of two components: first, a primary myelinated afferent fiber-mediated A response with a latency of 5–10 ms, and second, the unmyelinated fiber-mediated C response with latency of >100 ms. Given that primary afferent C fibers conduct nociceptive information, this study focused on the change in C responses.
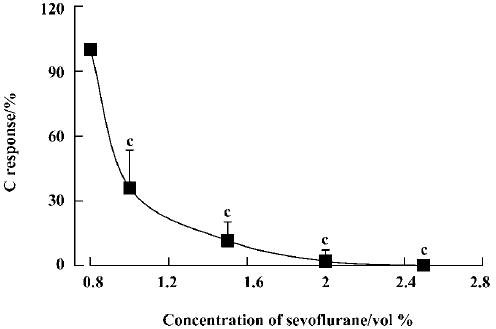
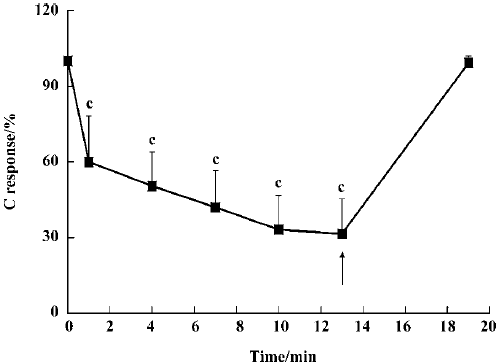
Effect of sevoflurane on the spinal cord To examine whether inhalation of sevoflurane affects the spinal cord neurons, the spinal cord was transected at the T9–10 segment. Following inhalation of sevoflurane at concentrations of 1.0%, 1.5%, 2.0%, and 2.5%, C responses were reduced to 36.1%±17.4%, 11.7%±8.6%, 2.2%±5.2% and 0% of control (P<0.01), respectively (Figure 1). The inhibition occurred in a time-dependent manner. When 1.0% sevoflurane was inhaled, C responses were 60.0%±18.1%, 50.4%±13.5%, 42.0%±14.5%, 33.2%±13.5%, and 31.5%±13.8% at 1 min, 4 min, 7 min, 10 min, and 13 min after inhalation, respec-tively, compared with the group without sevoflurane (Figure 2). C responses recovered almost completely within 9 min of ceasing inhalation.
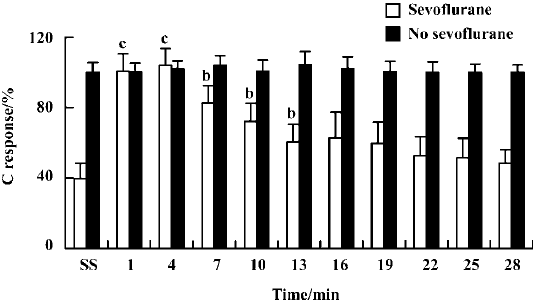
Reversal of sevoflurane-induced inhibition by bicuculline and naloxone As shown in Figure 3, bicuculline (0.1 mg/kg, iv) failed to alter the C response in the absence of sevoflurane. Following 20 min of inhalation of 1.0% sevoflurane, bicuculline at the same dose significantly increased the C responses at the time points of 1 min, 4 min, 7 min, 10 min, and 13 min (P<0.05). However, phaclofen (0.6 mg/kg) failed to alter the C response in the absence or presence of sevoflurane.
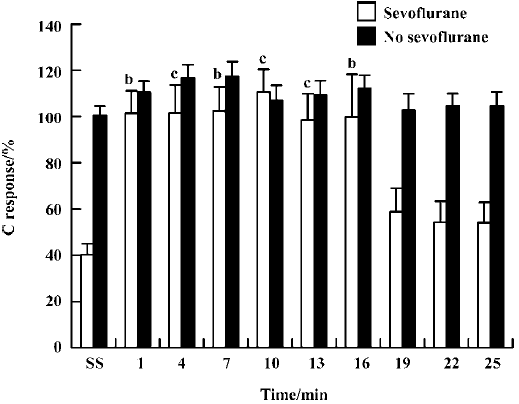
Although the C responses appeared to be slightly enhanced by naloxone alone (0.4 mg/kg, iv) in the absence of sevoflurane, this effect was not statistically different from the control (Figure 4). After the rats inhaled 1% sevoflurane, naloxone significantly increased their C responses at time points of 1 min, 4 min, 7 min, 10 min, 13 min, and 16 min (P<0.05).
Discussion
Several studies have shown that the mechanisms underlying the suppression of noxious stimuli-induced motor reactions by volatile anesthetics, including sevoflurane, are likely to be spinal in nature rather than involving superspinal structures[5,6,12]. Apart from immobility mediated by reducing the activity of spinal α-motoneurons, studies on the effects of sevoflurane on nociceptive sensory neurons in the spinal cord are generally lacking. The C-fiber-induced response recorded in the peripheral nerve or muscle is commonly used to characterize spinal processing and is considered to be an acute pain model[11]. By means of this model, the present work for the first time shows that inhalation of sevoflurane produces a dose-dependent reduction of primary sensory afferent C-fiber-mediated nociceptive responses in the rat after transection of the spinal cord at T9–10. This indicates that sevoflurane not only suppresses excitation in α-motor neurons but also inhibits the activity of pain-sensitive neurons in the dorsal horn of the spinal cord, in the absence of supraspinal input.
It is well-known that GABA receptors exist in the spinal cord and, when activated, cause decreased excitability of the neurons in which they exist[13]. A recent report showed that the intrathecal administration of GABAA or glycine receptor antagonists increased the MAC (minimum alveolus concentration) value of isoflurane by approximately 40% in rats[14]. Yamauchi et al also observed an approximately 50% reversal of halothane depression[15]. Similarly, in the present study, the sevoflurane-induced inhibition of nociceptive C responses was reversed by the intravenous administration of 0.1 mg/kg bicuculline, a GABAA receptor antagonist. This reversal effect by bicuculline seems unlikely to be involved in a release of a GABA-mediated tonic inhibition, given that 0.1 mg/kg bicuculline in the absence of sevoflurane did not increase nociceptive C responses significantly. Previous studies have revealed that the sevoflurane-induced inhibition of field potentials evoked from CA1 of the hippocampus is partially antagonized by phaclofen, a competitive antagonist of the GABAB receptor[16]. However, the antinocifensive effect of halothane was not affected by the intrathecal administration of GABAB receptor antagonist CGP 35348[7]. Similarly, our preliminary results show that intravenous administration of 0.6 mg/kg phaclofen failed to affect nociceptive C responses (data not shown). Taken together, these results suggest that the antinociceptive effect of sevoflurane might be mainly mediated by GABAA receptors in the spinal cord rather than GABAB receptors.
Opioid receptors are densely localized presynaptically and postsynaptically on pain-sensitive neurons in the spinal cord, which play a critical role in spinal antinociception. It has been reported that intravenous or intrathecal morphine is able to reduce the minimum alveolar concentration of halothane[8–10]. In the present study, the fact that naloxone blocked the sevoflurane-induced inhibition of nociceptive C responses provides further support for the involvement of spinal opioid receptors in antinociception mediated by sevoflurane.
In conclusion, the volatile anesthetic sevoflurane can directly depress spinal transmission of nociceptive information in the spinal cord, by a mechanism that may involve GABA receptors and opioid receptors.
Acknowledgement
We thank Christopher J LINGLE (Washington University, Saint Louis, Missouri, USA) for helpful discussions and editing this paper.
References
- Jones MU, Brooks PA, Harrison NL. Enhancement of gamma-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurons by three volatile anaesthetics. J Physiol (Lond) 1992;449:279-93.
- Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci 1999;55:1278-303.
- Franks NP, Lieb WR. Which molecular targets are most relevant to general anaesthesia? Toxicol Lett 1998;100-101:1-8.
- Bernd A. Different actions of general anaesthetics on the firing patterns of neocortical neurons mediated by the GABAA receptor. Anaesthesiology 1999;91:500-11.
- Rampil IJ, King BS. Volatile anaesthetics depress spinal motor neurons. Anaesthesiology 1996;85:129-34.
- Kammer T, Rehberg B, Menne D, Wartenberg HC, Wenningmann I, Urban BW. Propofol and sevoflurane in subanaesthetic concentrations act preferentially on the spinal cord. Anaesthesiology 2002;97:1416-25.
- Mason P, Owens CA, Hammond DL. Antagonism of the antinocifensive action of halothane by intrathecal administration of GABAA receptor antagonists. Anaesthesiology 1996;84:1205-14.
- Schwieger IM, Klopfenstein CE, Forster A. Epidural morphine reduces halothane MAC in humans. Can J Anaesth 1992;39:911-4.
- Drasner K, Bernards CM, Ozanne GM. Intrathecal morphine reduces the minimum alveolar concentration of halothane in humans. Anaesthesiology 1988;69:310-2.
- Criado AB, Gomez de Segura IA, Tendillo FJ, Marsico F. Reduction of isoflurane MAC with buprenorphine and morphine in rats. Lab Anim 2000;34:252-9.
- Bai L, Zhao ZQ. Ketamine-induced peripheral analgesia in rats. Acta Pharmacol Sin 1997;18:377-9.
- Antognini JF, Carstens E, Buzin V. Isoflurane depresses motoneuron excitability by a direct spinal action: an F-wave study. Anaesth Analg 1999;88:681-5.
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol 1991;36:35-92.
- Zhang Y, Wu S, Eger I II, Sonner JM. Neither GABAA nor strychnine-sensitive glycine receptors are the sole mediators of MAC for isoflurane. Anaesth Analg 2001;92:123-7.
- Yamauchi M, Sekiyama H, Shimada SG, Collins JG. Halothane suppression of spinal sensory neuronal responses to noxious peripheral stimuli is mediated, in part, by both GABAA and glycine receptor systems. Anaesthesiology 2002;97:412-7.
- Hirota K, Roth SH. Sevoflurane modulates both GABAA and GABAB receptors in area CA1 of rat hippocampus. Br J Anaesth 1997;78:60-5.
