Involvement of nociceptin/orphanin FQ in release of hypothalamic GnRH mediated by ORL1 receptor in ovariectomized rats
Introduction
Nociceptin/orphanin FQ (OFQ) is a heptadecapeptide that has high amino acid sequence homology to the endogenous opioid peptides, especially dynorphin A[1,2]. OFQ is the putative endogenous ligand for the opioid receptor-like1 receptor (ORL1 receptor)[2,3]. The ORL1 receptor has structural and functional homology with the δ, κ, and μ classic opioid receptors. Despite its close similarity to opioid receptors, the ORL1 receptor does not selectively bind opioids and opioids antagonists[3]. A number of physiological effects of OFQ have been reported, including nociceptive modulation, and cardiovascular and renal physiological functions[2,4,5].
The classic opiates are involved in the regulation of anterior pituitary hormone secretion. Opiate administration increases prolactin and growth hormone[6], while decreasing the release of luteinizing hormone in a naloxone-reversible manner[7]. The homology between the opiate peptides and OFQ as well as between their receptors, coupled with the localization of OFQ in the hypothalamus suggest a neuroendocrine role for OFQ[8,9].
GnRH, as an important hypothalamic decapeptide, plays a key role in the functions of the hypothalamic-pituitary-ovary axis (HPOA) by modulating the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary[10]. Endogenous opioid peptides, particularly β-endorphin, constitute an important inhibitory component of the neural circuitry that regulates GnRH secretion and a significant decrease in the inhibitory opioid tone is critical for the generation of a LH surge during the estrous cycle[11].
OFQ and the ORL1 receptor are densely localized in the preoptic area (POA), ventralmedial hypothalamus (VMH) and arcuate nucleus (ARC), all of which have been identified as the chief hypothalamic nuclei regulating GnRH release[8]. It has been demonstrated that OFQ activates the G protein-coupled inwardly rectifying K+ channel (GIRK) and hyperpolarizes many hypothalamic neurosecretion cells, especially in the ARC[12]. Recent studies have revealed that OFQ potently and dose-dependently inhibits forskolin-induced GnRH release from rat hypothalamic fragments[13]. We postulated that OFQ might play a role in the regulation of GnRH release in rat hypothalamus. To test the hypothesis, the effects of the central administration of OFQ on rat hypothalamus GnRH release and plasma LH levels were observed in the present study.
Materials and methods
Animals and drugs Female Sprague-Dawley rats weighing 200–220 g obtained from the Animal Center of Shanghai Medical School of Fudan University had both ovaries surgically removed under aseptic conditions. They were kept in an air-conditioned room with controlled lighting (light 12 h:dark 12 h) and given free access to laboratory chow and tap water. Experiments were carried out 4–6 weeks after the ovariec-tomies. Nociceptin/orphanin FQ (MW 1810) and [Nphe1]NC(1-13)NH2 (NC13; MW=1382) were purchased from Phoenix Pharmaceutical Company (St Joseph, MD, USA). The compounds were dissolved in artificial cerebrospinal fluid (ACSF; 128 mmol/L NaCl, 2.5 mmol/L KCl, 1.4 mmol/L CaCl2, 1.0 mmol/L MgCl2 and 1.2 mmol/L Na2HPO4; pH 7.4)[14]. All other reagents and solvents were of analytical grade.
Incubation of hypothalamus slices Animals were killed by decapitation in a period of less than 30 min and medial basal hypothalamus slices were quickly dissected at 4 ºC as described by Lamar et al[15]. The slices were placed into 2 mL ice-cold ACSF in a chamber made from a 5 mL plastic syringe specially modified for incubation. The slices were maintained at 37 ºC in an atmosphere of 5% CO2 and 95% O2 with gentle agitation for 30 min preincubation. The slices then were treated with ACSF or 0.2, 2, and 20 mmol/L OFQ and incubated for 2 h. Every 30 min, 200 µL medium was extracted, then 200 µL fresh ACSF was added to the system. The incubation medium was collected in tubes and stored at -20 ºC for GnRH radioimmunoassay.
Push-pull perfusion Animals were anesthetized with sodium pentobarbital (40 mg/kg, ip) and placed on a stereotaxic instrument. A push-pull cannula with a removable inner stylette constructed in our laboratory was implanted towards the hypothalamus POA (Bregma AP: 0.4 mm; L: 0.8 mm; H: 8.5 mm)[16]. The device was fixed onto the rat skull with an anchor screw and dental cement, and the animals were given a minimum recovery period of 7 d. On the day of perfusion, the inner stylette was removed and replaced with the inner cannula perfusion assembly. ACSF was infused through the push cannula and collected from the pull cannula at a flow rate of 10 µL/min. After a 30 min equilibration period, 0.02, 0.2, or 2 mmol/L OFQ solution was perfused. The perfusate was collected every 20 min over a total period of 2 h and stored at -80 ºC for GnRH radioimmunoassay.
Intracerebroventicular injection Implantation of the cannula was performed stereotaxically under anesthesia with sodium pentobarbital (40 mg/kg, ip). Stereotaxic surgical procedures were used to implant one 22 gauge stainless steel guide cannula with a removable 28 gauge inner stylette to the left lateral ventricle (Bregma AP: 1.0 mm; L: 1.5 mm; H: 3.0 mm)[16]. Experiments with icv injection were performed at least 7 d after operation. The OFQ solution was added with protease inhibitor (1 g/L) for preventing proteolysis after injection. Two, 20 or 200 nmol OFQ dissolved in 10 µL ACSF was infused through a 28 gauge cannula extended 0.5 mm beyond the guide cannula. The needle was connected to a 10-µL syringe by a polyethylene tube and the drug solutions were delivered by infusion pump at a flow rate of 5 µL/min. The injection needle was maintained in place for an extensive period of 10 min after the injection. Under anesthesia conditions, OVX rats were injected with ACSF, OFQ (2, 20, or 200 nmol OFQ in 10 µL ACSF) or NC13 (20, 50, 100, and 200 nmol NC13 in 10 µL ACSF). Blood samples of 400 µL were withdrawn from the rat tail vein every hour over a total period of 4 h after icv injection. Blood plasma (200 µL) was separated from each blood sample by centrifugation and stored at -20 °C until LH radioimmunoassay.
RT-PCR analysis Total cytoplasmic RNA was isolated from the medial basal hypothalamus, hippocampus and pituitary of ovariectomized rats using the Trizol reagent (Life Technologies, Rockville, MD, USA). Total RNA (4 µg) was digested with DNase RNase-free enzyme to eliminate genomic DNA, and then converted to complementary DNA (cDNA) using 200 U Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) in 20 µL of buffer containing 0.4 mmol/L deoxynucleotide triphosphates, 20 U RNase inhibitor and 0.8 µg oligo (deoxythymidine)15 (Sino-American Biotechnology Company, Shanghai, China). Specific primers were 5'-CAGGCTGTTAATGTGGCCATATG-3' and 5'-GAGCCTGAAAGCAGACGGACACC-3' (synthesized in Shanghai Sangon Biological Engineering Technology and Service Company), which annealed to bases 493–515 and 743–721 of the ORL1 receptor[17]. Twenty-eight cycles under the following PCR conditions were carried out: denaturation at 94 °C for 45 s, annealing at 60 °C for 45 s and elongation at 72 °C for 60 s with Thermus aquaticus DNA polymerase (Promega, Medison, WI, USA). The RT-PCR products (5 µL) were electrophoresed in a 1.6% agarose ethidium bromide gel and visualized using the SYNGENE imaging system (GeneSnape Software, London, UK).
GnRH and LH radioimmunoassay GnRH in perfusate was assayed by using a commercial RIA kit (Sigma) and measurements were performed according to the manufacturer’s instructions. Plasma LH was measured by using an LH RIA kit (Shanghai Institute of Biologic Products, Shanghai, China), and the sensitivity of the assay was 0.4 pg/mL. The intra-assay coefficient of variation was 3.5% and the intro-assay variation was 4.8%.
Statistical analysis All results are expressed as mean±SD and were analyzed by the Statistical Package for the Social Sciences (SPSS) statistical software. The data in Figures 1–3 were analyzed by using a two-way repeated measures analysis of variance (ANOVA) with time as the repeated measure. The other results were analyzed by one-way ANOVA and the significance of difference was determined by the Newman-Keuls test. When only two treatment groups were being compared, Student’s t-test was used. A probability of P<0.05 was considered to be statistically significant.
Results
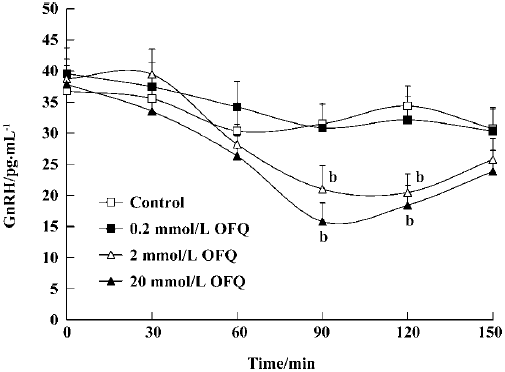
Effect of OFQ on GnRH release from hypothalamus slices It was apparent that GnRH release began to decrease 60 min after OFQ administration. In the 2 mmol/L and 20 mmol/L OFQ groups, GnRH concentrations in the medium were significantly decreased (P<0.05) at 90 min (21.01±3.77 pg/mL and 15.78±3.02 pg/mL) after OFQ administration compared with the control group at the same time (31.49±3.34 pg/mL). OFQ at a concentration of 0.2 mmol/L did not affect GnRH release during the 120 min incubation time (Figure 1).
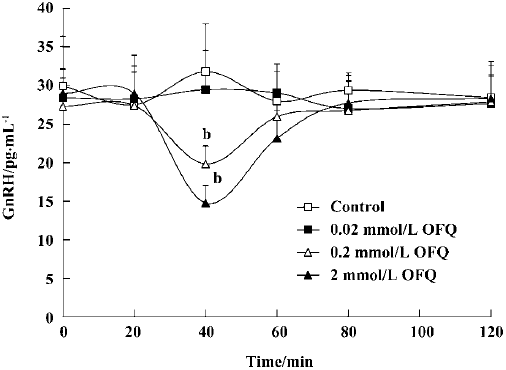
Effect of OFQ on GnRH release from POA OFQ evoked a dose-dependent inhibitory effect on POA GnRH release in ovariectomized rats (P<0.05). GnRH release stayed at approximately the same level over 120 min in the control group. The administration of 0.2 mmol/L and 2.0 mmol/L OFQ caused a significant decrease in GnRH release at 40 min (19.83±2.35 pg/mL and 14.77±2.31 pg/mL, respectively), which lasted approximately 20 min, as compared with the control group (31.79±6.19 pg/mL). OFQ at a concentration of 0.02 mmol/L had no significant effect on GnRH release over 120 min (Figure 2).
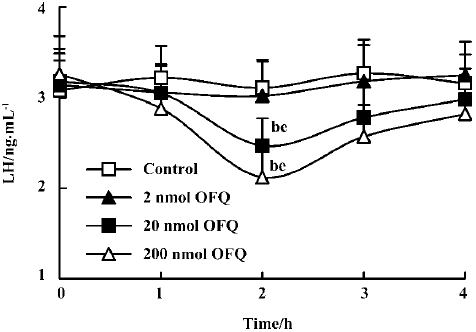
Effect of icv injection of OFQ and NC13 on plasma LH levels A dose-dependent decreasing effect on plasma LH levels was observed 2 h after icv injection of OFQ. The plasma LH levels in the control group ranged from 3.08±0.33 ng/mL to 3.27±0.37 ng/mL and were not significantly different from those of the 2 nmol OFQ group at any time point over 4 h. Administration of 20 nmol and 200 nmol OFQ significantly decreased pituitary LH secretion (P<0.05). The strongest effect was achieved at 2 h after injection in the 20 nmol and 200 nmol OFQ groups (2.47±0.30 ng/mL and 2.12±0.32 ng/mL), as compared with the control group (3.11±0.31 ng/mL). The LH decrease occurred at 2 h after injection for both doses and persisted until 3 h (Figure 3).
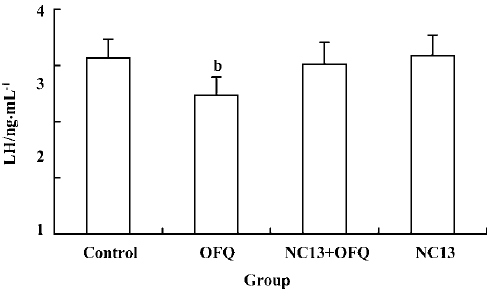
A significant decrease in plasma LH level (2.47±0.30 ng/mL) induced by 20 nmol OFQ was abolished by pretreatment with 20 nmol NC13, a competitive antagonist of the ORL1 receptor (3.02±0.39 ng/mL). No change in plasma LH level was observed with a single icv injection of 20 nmol NC13 (Figure 4).
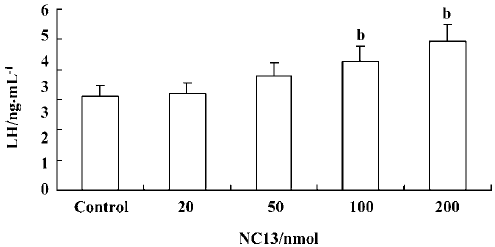
Effect of icv injection of NC13 at different doses on plasma LH levels The plasma LH levels in the 20 nmol and 50 nmol NC13 groups (3.18±0.36 ng/mL and 3.78±0.46 ng/mL) were not significantly different from those of the control group (3.13±0.34 ng/mL) 2 h after administration. Inter-estingly, 100 nmol and 200 nmol NC13 stimulated a significant increase in plasma LH levels (4.27±0.51 ng/mL, 4.95±0.53 ng/mL) 2 h after icv infusion (P<0.05) and the effect was dose-dependent. This reveals that a single high-dose icv injection of NC13 produces a significant increase in plasma LH levels in OVX rats (Figure 5).
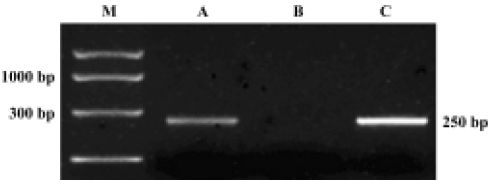
RT-PCR analysis RT-PCR analysis to check for ORL1 receptor expression revealed that the ORL1 receptor transcript was expressed in the hypothalamus and hippocampus of OVX rats, but not in the pituitary (Figure 6).
Discussion
OFQ and its receptor are densely distributed in several nuclei in the hypothalamus, suggesting that OFQ might mediate and modulate hypothalamic functions[2,8]. There is evidence that OFQ is involved in the regulation of some important hypothalamic functions, such as feeding behavior, temperature control and sexual behavior[18–20]. OFQ has been shown to stimulate prolactin and growth hormone secretion in male and female rats as an endogenous opioid peptide[21]. Our results show that central administration of OFQ can inhibit the release of GnRH in the hypothalamus in vitro and in vivo and decrease the plasma LH level, suggesting that OFQ might play a role in the modulation of hypothalamic GnRH secretion and pituitary LH release. Recent data from our laboratory and from other investigators indicate a neuroendocrine role for OFQ in mechanisms of the hypothalamus governing reproduction[13,21].
It is known that GnRH is the key central regulator in the estrous cycle. Despite intensive investigation, the mechanism underlying GnRH secretion remains poorly understood[10]. GnRH cell bodies form a loose continuum from the medial septum to the ventral anterior hypothalamus. GnRH is released from nerve terminals in the median eminence, where it is transported to the anterior pituitary to act on gonadotropes to stimulate LH and FSH secretion to the blood[22]. The ovariectomy performed in our experiments was intended to remove the effects of the estrous cycle on the hypothalamus GnRH and plasma LH secretion in female rats. Our results show that 2 mmol/L and 20 mmol/L OFQ inhibits GnRH release from rat medial basal hypothalamus slices in a dose-dependent manner, which is in agreement with previous reports[13]. Moreover, by using push-pull perfusion, we demonstrated that OFQ could also inhibit GnRH release from rat hypothalamus POA in vivo, further indicating the pharmacological effect of OFQ on hypothalamic GnRH release.
It was observed in our study that central injection of 20 nmol OFQ significantly decreased plasma LH levels, and the strongest effects occurred 2 h after administration. Pretreatment with 20 nmol selective ORL1 receptor antagonist NC13 can reverse the inhibitory effect that is induced by icv injection of 20 nmol OFQ. These results further confirm that the decrease of pituitary LH secretion induced by OFQ administration is mediated by the ORL1 receptor in the brain. We found that the ORL1 receptor was not expressed in rat pituitary by using RT-PCR analysis, indicating that OFQ might have no direct effect on pituitary hormone secretion. There has been no evidence of OFQ receptors on the anterior pituitary gland until now. These results strongly suggest that by interacting with its own receptor, the ORL1 receptor, OFQ elicits its inhibitory effect on hypothalamus GnRH release, thus decreasing the plasma LH level.
Time course studies reveal that the strongest effect of OFQ on GnRH release occurred at 40 min after OFQ administration in vivo, but it takes 2 h before any significant effects are seen on LH levels. The apparent discrepancy is probably due to the interval required by neuroendocrine transduction. The neural signal of GnRH decrease evoked by OFQ is transduced from the medial basal hypothalamus to the median eminence and then to the anterior pituitary, resulting in a decrease of LH secretion. Likewise, the time discrepancy in these results could also offer support for this postulation.
The effect of OFQ on GnRH secretion is likely to be due to an indirect mechanism of action, because OFQ has no such inhibitory effect when applied directly to immortalized GnRH (GT-1) neurons in vitro[13]. There is accumulating evidence that OFQ is able to inhibit the K+-evoked release of glutamate and suppress glutamate transmission in the central nervous system of various species[23–25]. There is a general agreement that the glutamatergic pathway is a major excitory element in the regulation of GnRH release [26]. We speculate that hypothalamic glutamate might mediate the inhibitory effects of OFQ on GnRH secretion. Therefore, we are currently investigating the effects of NMDA or non-NMDA antagonists on the decrease of GnRH and LH secretions induced by OFQ. Undoubtedly, our hypothesis needs to be supported by more experimental evidence, and at this stage we could not exclude the possibility that OFQ has a direct effect on GnRH neurons in vivo.
Several studies have indicated that OFQ has an anti-opioid effect in nociceptive modulation because OFQ can induce hyperalgesia and antagonize opioid analgesia mediated by the μ and δ receptors in the brain[27,28]. Morphine and endogenous opioid peptides such as β-endorphin decrease the release of GnRH and LH in a naloxone-reversible manner[7,29]. Our data suggest that OFQ also plays a negative neuroendocrine role in the modulation of GnRH and LH release as classic opioids.
As the first identified selective ORL1 receptor antagonist, NC13 has allowed us to characterize some biological effects that are clearly mediated through the ORL1 receptor in vitro and in vivo[4]. It is shown in our work that NC13 can antagonize the inhibitory effect of OFQ on LH secretion. Neverthe-less, we also observed that icv injection of 100 nmol and 200 nmol NC13 alone enhanced the plasma LH levels of ovariectomized rats. The present study reveals an unexpected function of NC13, that is, it can also act as a potent stimulator of LH release at a higher concentration. This suggests that OFQ has a tonic physiological role in the control of pituitary LH secretion. Our next study will focus on elucidating the mechanism for the involvement of the endogenous OFQ/ORL1 receptor system in regulating GnRH and LH release in male and female rats.
In summary, these results demonstrate that central administration of OFQ might inhibit the release of hypothalamic GnRH and decrease the level of plasma LH via the ORL1 receptor in OVX rats. In addition, our work implies that hypothalamic OFQ might participate in the neuroendocrine regulation of GnRH and LH secretion in female rats. Although the neuroendocrine role of OFQ remains to be explored more directly, our results provide evidence for novel mechanisms for hypothalamic OFQ regulation of GnRH and LH secretion.
References
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsem RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science 1995;270:792-4.
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995;377:532-5.
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, et al. ORL1, a novel member of the opioid receptor family: cloning, functional expression and localization. FEBS Lett 1994;341:33-8.
- Calo G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol 2000;129:1261-83.
- Kapusta DR. Neurohumoral effects of orphanin FQ/nociceptin: relevance to cardiovascular and renal function. Peptides 2000;21:1081-99.
- Janik J, Callahan P, Rabii J. The role of µ1 opiate receptor subtype in the regulation of prolactin and growth hormone secretion by β-endorphin in female rats: Studies with naloxonazine. J Neuroendocrinol 1992;4:701-8.
- Meites J, Bruni JF, Vanvugt D, Smith AF. Regulation of endogenous opioid peptides and morphine to neuroendocrine function. Life Sci 1977;24:1325-30.
- Neal CR, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system. J Comp Neurol 1999;406:503-47.
- Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand nociceptin/orphanin FQ. Trends Pharm Sci 1997;18:293-300.
- Herbison AE. Multimodal influence of estrogen upon gonadotropin releasing hormone. Endocr Rev 1998;19:302-30.
- Kalra SP. Mandatory neuropeptide-steroid signaling for the preovulatory luteinizing hormone-releasing hormone discharge. Endocr Rev 1993;14:507-37.
- Wanger EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology 1998;67:73-82.
- Dhandapani KM, Brann DW. Orphanin FQ inhibits GnRH secretion from rat hypothalamic fragments but not GT1-7 neurons. Neuroreport 2002;13:1247-9.
- Watanobe H, Sasaki S, Takebe K. Evidence that intravenous administration of interleukin-1 stimulates corticotropin releasing hormone secretion in the median eminence of freely moving rats: estimation by push-pull perfusion. Neurosci Lett 1991;133:7-10.
- Lamar CA, Bhat GK, Mahesh VB, Brann DW. Evidence that neuronal oxide synthase but not heme oxygenase increases in the hypothalamus on proestrous afternoon. Neuroendocrinology 1999;70:360-7.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. New York: Academic Press; 1998.
- Briscini L, Corradini L, Ongini E, Bertorelli R. Up-regulation of ORL1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 2002;447:59-65.
- Olszewski PK, Levine AS. Characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology 2004;145:2627-32.
- Chen XH, McClatchy DB, Geller EB, Liuchen LY, Tallarida RJ, Adler MW. Possible mechanism of hypothermia induced by intracerebroventricular injection of orphanin FQ/nociceptin. Brain Res 2001;904:252-8.
- Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport 1997;22:3857-60.
- Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone secretion in male and female rats. Brain Res 1998;802:228-33.
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction 2001;122:1-10.
- Nicol B, Rowbotham DJ, Lambert DG. Nociceptin/orphanin FQ inhibits glutamate release from rat cerebellar and brain stem slices. Neurosci Lett 2002;326:85-8.
- Meis S, Pape HC. Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J Physiol 2001;532:701-12.
- Schlicker E, Morari M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 2000;21:1023-9.
- Jennes L, Lin W, Lakhlani S. Glutamatergic regulation of gonadotropin-releasing hormone neurons. Prog Brain Res 2002;141:183-92.
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience 1996;75:333-7.
- Zhu CB, Zhang XL, Xu SF, Cao XD, Wu GC, Li MY, et al. Antagonistic effect of orphanin FQ on opioid analgesia in rat. Acta Pharmacol Sin 1998;19:10-4.
- Ching M. Morphine suppresses the proestrous surge of GnRH in pituitary portal plasma of rats. Endocrinology 1983;112:2209-21.
