Enhancement of binding activity of soluble human CD40 to CD40 ligand through incorporation of an isoleucine zipper motif1
Introduction
CD40, a 50 kD type I transmembrane glycoprotein, is a member of the tumor necrosis factor receptor (TNFR) superfamily[1]. It is expressed on antigen-presenting cells (APC) including B cells, dendritic cells, and macrophages. The interaction of CD40 with its cognate ligand, CD40L (CD154), which is expressed mainly on activated CD4+ T cells, is absolutely required for primary immune response and accompanying inflammatory responses[2,3]. Recognition of the specific peptide antigen in the context of major histocompatibility complex (MHC) on APC by the CD4+ T cells leads to the transient expression of CD40L, which interacts with CD40, resulting in the upregulation of accessory molecules such as CD80 and CD86 on the APC. CD80/CD86 in turn provide sufficient co-stimulatory signals for the successful activation of naive T cells and differentiation to effector cells[1,3]. Therefore, CD40-CD40L interactions are crucial in the priming, expansion, and maturation of CD4+ T cells. The development of adapted immune response is dependent on the signaling of CD40 by CD40L[1].
It has been well documented that blockade of the CD40-CD40L interaction can protect against the progression of antibody- and cell-mediated autoimmune diseases, and reduce allograft rejection, thus prolonging graft survival, even engendering long-lived antigen-specific tolerance[4–8]. Blockade of the CD40-CD40L interaction is usually achieved by using anti-CD40L monoclonal antibodies (mAb)[4–6]. It has also been shown that soluble CD40-immunoglobulin Fc fusion protein (CD40Ig) can block the interaction of CD40 with its ligand[7,8].
The isoleucine zipper (IZ) is a trimerization motif that forms trimers spontaneously in solution. Although the incorporation of an IZ motif[9–11] can enhance the binding activity of TNF superfamily members such as CD40L[12] and FasL[13], it is still unknown whether trimerization of a soluble CD40 could enhance binding activity to CD40L. In the present study, we demonstrated that the recombinant soluble CD40 containing an IZ motif could spontaneously form trimers during refolding and had enhanced binding activity for CD40L, which was expressed on the cell surface.
Materials and methods
Construction of expression plasmids for human CD40 derivatives To construct the expression plasmid for human soluble CD40 with a hexa-His tag (designated as shCD40His), the DNA fragment coding for hexa-His and a Gly-Thr linker were fused to the 3'-terminus of the cDNA fragment coding for the CD40 isoform II (1–185)[14] by polymerase chain reaction (PCR) with the previously cloned CD40 cDNA fragment (GenBank accession number AY225405) as a template. The PCR product was digested with NdeI and BamHI and cloned into the pET-3c vector (Novagen, Madison, WI, USA) containing the T7 promoter for expression in E coli. To construct the other expression plasmid for the shCD40 containing an IZ trimerization domain[9–11] fused to the carboxyl terminus of CD40 isoform II[14] and hexa-His tag (designated shCD40IZ), the DNA fragments encoding the hexa-His tag and isoleucine zipper sequences were fused to the sequence of CD40 isoform II and ligated into the pET-3c vector. The shCD40IZ molecule includes a Gly-Gly-Gly-Ser sequence at the junction between the CD40 region and the IZ domain. All constructs were verified by DNA sequencing. The predicted products of these 2 expression plasmids, shCD40His and shCD40IZ, are shown in Figure 1.
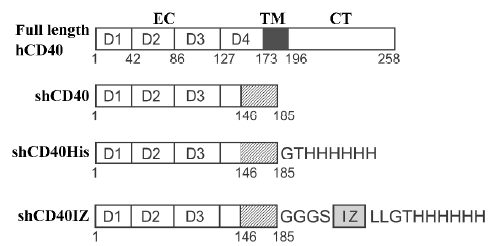
Expression and purification of shCD40His and shCD40IZ The expression of the CD40 derivatives in E coli strain BL21(DE3) (Novagen) was induced by 0.4 mmol/L isopropylthio-β-D-galactoside (IPTG). The bacterial cells from 1 L culture were then harvested by centrifugation, and insoluble protein aggregates (inclusion bodies) were purified as previously described[15]. In brief, the cell pellet was re-suspended in 50 mmol/L Tris-HCl buffer (pH 8.0) containing 1 mmol/L ethylenediamine tetraacetic acid (EDTA), 0.1 mmol/L phenylmethylsulfonyl fluoride and 10 mmol/L dithiothreitol (DTT), and then sonicated on ice. The insoluble pellet was collected by centrifugation and washed with washing buffer [50 mmol/L Tris-HCl (pH 8.0), 100 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT and 5 g/L Triton X-100], followed by 3 rounds of sonication, centrifugation, and collection. The pellet was washed three 3 times with washing buffer without Triton X-100. The refolding of CD40 derivatives was carried out by dilution as described elsewhere[16]. The shCD40His was purified by native Ni-NTA resin (Invitrogen, Carlsbad, CA, USA) affinity chromatography, whereas shCD40IZ was purified by size exclusion chromatography with a Sephadex G-150 column equilibrated with 20 mmol/L Tris–HCl (pH 8.0) containing 150 mmol/L NaCl. The purified protein was concentrated by ultrafiltration and changed into 50 mmol/L Tris-HCl buffer (pH 8.0). The protein concentration of the purified products was determined by measuring A280 nm and A260 nm, and calculated according to the following empirical formula: 1.45×A280 nm–0.74×A260 nm=protein concentration in g/L.
Sodium dodecylsulfate-polyacrylamide gel electrophoresis and Western blotting Western blotting was performed using sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 15%) according to Laemmli’s discontinuous system[17]. After electrophoresis, the gels were either stained with Coomassie brilliant blue R250 or blotted onto nitrocellulose paper (Bio-Rad, Hercules, CA, USA) for Western blotting with the rabbit antihuman CD40 antibody (H-120; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-His (C-term) mAb (Invitrogen, Carlsbad, CA, USA).
Gel filtration analysis The molecular size of shCD40IZ and shCD40His was determined by gel-filtration chromatography in a column (2 cm×100 cm) packed with Sephadex G-150 (Amersham-Pharmacia, Uppsala, Sweden) equilibrated with 20 mmol/L Tris-HCl (pH 8.0) containing 150 mmol/L NaCl and eluted with the same buffer at a flow rate of 0.5 mL/min. Absorbance was monitored at 280 nm, and 2.0 mL fractions were collected. Gel filtration molecular weight markers (MW-GF-200, Sigma) were loaded onto the same column and their elution volumes were used to estimate the relative molecular size of 2 CD40 derivatives.
Cell culture The Jurkat T cells (American Type Culture Collection number: TIB-152, Rockville, MD, USA) were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) and incubated in a humidified atmosphere with 5% CO2 at 37 °C. Cells were subcultured every 3 d before reaching con-fluency so that actively growing cells were used for experi-ments.
Binding assay Binding of CD40 derivatives to CD40L expressed on Jurkat T cells was determined by flow cyto-metry. Briefly, 2×106 Jurkat T cells stimulated with PHA (10 µg/mL) for 24 h were collected and resuspended in phosphate-buffered saline (PBS) containing 10 g/L bovine serum albumin (BSA). The cells were first incubated at 4 °C with shCD40His or shCD40IZ (0.08–50 µg/mL) or without the recombinant proteins as control. After 30 min, the cells were washed and further incubated at 4 °C for 30 min with mouse anti-His (C-term) mAb. After washing, the cells were incubated with phycoerythrin (PE)-conjugated rabbit F(ab')2 anti-mouse IgG (DakoCytomation, Denmark) at 4 °C for 30 min. After the final incubation, the cells were washed with PBS twice and fixed with paraformaldehyde (4% in PBS). Ten thousand events were analyzed on a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA, USA). The expression of CD40L on Jurkat T cells was also analyzed with FACSCalibur after the cells were stained with PE-anti-CD40L (PharMingen, San Diego, CA, USA).
Results
Expression and purification of the recombinant soluble CD40 derivatives The 2 soluble CD40 derivatives, shCD40His and shCD40IZ, constructed in this study are schematically depicted in Figure 1. After being constructed, the recombinant plasmids for CD40 derivatives were verified by DNA sequencing. Both were expressed at high levels in E coli after IPTG induction (Figure 2). The molecular masses of shCD40His and shCD40IZ were estimated to be 23 kDa and 27 kDa, respectively, which were comparable to their theoretical values. Both shCD40His and shCD40IZ were largely expressed in an insoluble form in inclusion bodies and thus each was first purified by isolation of the inclusion bodies with washing and centrifugation.
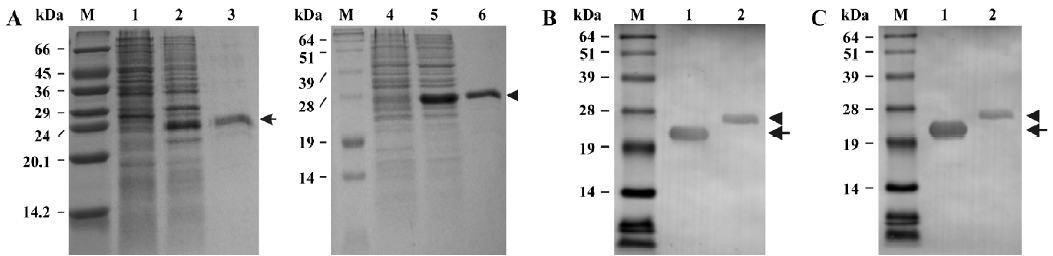
Both of the CD40 derivatives were refolded from denatured proteins by using the dilution method. shCD40His was further purified by Ni-NTA affinity chromatography to homogeneity because it mainly existed as a monomer, whereas shCD40IZ could be purified by gel filtration in order to eliminate the larger multimers formed during refolding. After purification, each soluble CD40 preparation was analyzed for purity using Coomassie gels (Figure 2A). The purity of both shCD40His and shCD40IZ was ≥95%.
Western blotting analysis of the recombinant soluble CD40 derivatives The soluble CD40 derivatives were detected by Western blotting using either anti-CD40 antibody (H-120) or anti-His (C-terminus) mAb. Both shCD40His and shCD40IZ could react with anti-CD40 antibody as expected. One specific band of the expected molecular weight was visualized in each lane for the purified products (Figure 2B). Furthermore, these 2 recombinant proteins could both react with anti-His mAb against a hexa-His tag at the carboxyl terminus (Figure 2C), indicating that the His-tag was available on the surface of the proteins, which provided a tag for detection of the recombinant proteins.
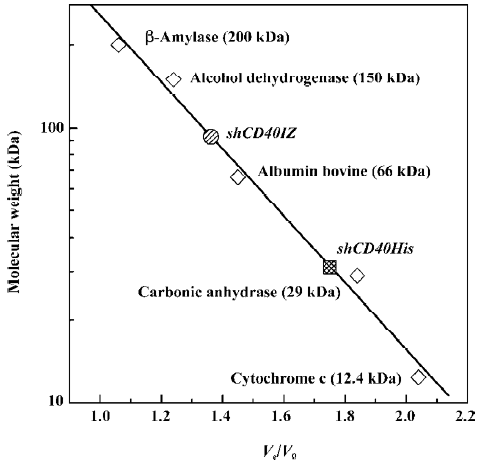
Gel filtration analysis of soluble CD40 derivatives To determine whether shCD40IZ existed as multimers, size exclusion chromatography was performed to evaluate its apparent molecular mass in solution. The apparent molecular mass of shCD40His was 32 kDa (Ve/V0=1.78), whereas that of shCD40IZ was 91 kDa (Ve/V0=1.34) (Figure 3). Given the molecular mass of the monomer polypeptides, shCD40IZ was most likely to be a trimer in solution, whereas shCD40His existed as a monomer.
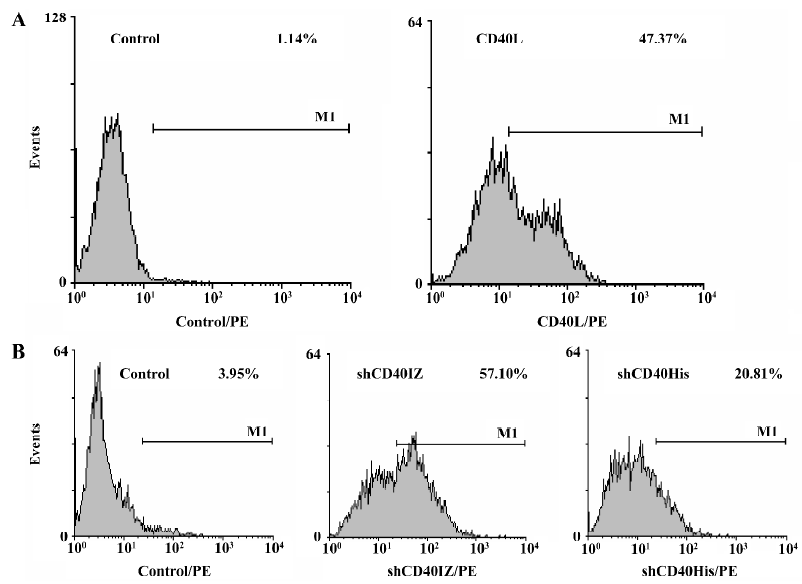
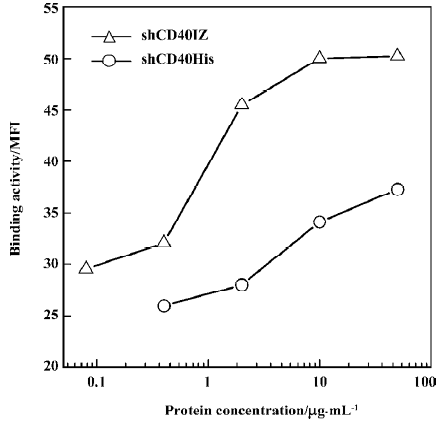
Binding activity of refolded CD40 derivatives with CD40L To determine the binding activity of soluble CD40 derivatives with their cognate ligand CD40L, flow cytometry was used to evaluate the binding of the recombinant proteins to PHA-stimulated Jurkat T cells. The expression level of CD40L detected by CD40L-specific mAb was 47.37% after PHA stimulation. Although the staining rate (20.81%) of 50 µg/mL shCD40His was much lower than that of the anti-CD40L antibody staining, the staining rate (57.10%) of 50 µg/mL shCD40IZ was comparable to that of the antibody staining (Figure 4). To further compare the binding activities of these 2 derivatives, the mean fluorescence intensity in flow cytometric analysis using different concentrations of these recombinant proteins was determined. shCD40IZ had much higher binding activity to its ligand (CD40L) than did shCD40His (Figure 5).
Discussion
Several studies have demonstrated that CD40L can form trimers both in the native state[18] and in the soluble form[19]. The presence of an IZ trimeric domain in soluble CD40L can enhance its biological activity, probably because the trimeric domain stabilizes the soluble CD40L trimer[12]. Natural soluble CD40 has also been found to form oligomers that have binding activity for CD40L, suggesting that it has a propensity to form oligomers[20]. Based on these observa-tions, we hypothesized that trimerization of soluble CD40 molecules might increase their binding activity for their cognate ligand, CD40L. In the present study, we aimed to investigate this hypothesis by incorporating an IZ trimerization domain to enhance the formation of soluble CD40 trimers.
The results presented here indicate that 2 soluble CD40 derivatives, shCD40His and shCD40IZ, which were both expressed in E coli and refolded from inclusion bodies, had significant binding activity for CD40L on the cell surface. Moreover, the binding activity of shCD40IZ was significantly higher than that of shCD40His. The enhancement of binding activity was most likely due to the incorporation of the IZ trimerization domain[9–11], which would lead to the formation of trimeric shCD40IZ because the IZ motif sequences spontaneously adopt trimeric conformations in the buffer solution[10]. As expected, our results showed that incorporation of an IZ trimerization domain resulted in the formation of trimeric shCD40IZ, whereas shCD40His, which lacked the IZ motif, existed in monomeric form, as demonstrated by size-exclusion chromatography. Hence, the IZ trimeric domain can stabilize the CD40 trimers in solution. Formation of trimeric shCD40IZ enabled a higher binding activity (ie increased its avidity) due to its multivalency. Therefore, we conclude that CD40 derivatives with an IZ motif can spontaneously form trimers, and that trimerization of CD40 derivatives results in enhanced binding activity with CD40L.
Our results are consistent with those of previously reported studies. One study showed that pentamerized CD40 had greatly increased avidity as compared with CD40Ig[21]. In another study, it was demonstrated that natural soluble CD40 might exist in a high molecular weight (150 kDa) form. It might be oligomerized molecules which are never found with recombinant soluble CD40. This natural soluble CD40 could bind to CD40L, whereas a recombinant soluble CD40 was unable to bind CD40L[20]. In the present study, however, both shCD40IZ and shCD40His could bind to CD40L, which suggests that the CD40 derivatives were correctly refolded for binding to their ligands. This is consistent with the CD40 3-dimensional model[22,23], which indicates that the ligand binding sites are mainly located within cysteine-rich domains 2 and 3, which were both retained in our constructs. In addition, the staining rate and fluorescence intensity of trimeric shCD40IZ for CD40L on Jurkat T cells were similar to those of anti-CD40L monoclonal antibody but much higher than those of shCD40His, suggesting that the affinity of shCD40IZ for CD40L was comparable to that of the specific antibody and much higher than that of shCD40His. Therefore, it is reasonable to postulate that this trimeric shCD40IZ could be used as a potential reagent for blocking CD40-CD40L interac-tions, although further research remains to be carried out to confirm this.
In conclusion, the presence of the IZ trimerization domain in the CD40 molecule stabilized the formation of trimers, leading to enhanced binding activity for its cognate ligand. Additional investigations into the in vitro and in vivo biological activities of the trimeric CD40 derivative should provide further evidence for its enhanced binding activity and assist in the development of an alternative therapeutic agent for blocking the CD40-CD40L interaction in place of the commonly used anti-CD40L monoclonal antibodies.
Acknowledgement
We thank Miss Li-rui DU for her excellent technical assistance with cell culture.
References
- Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol 2004;22:307-28.
- Xu Y, Song G. The role of CD40-CD154 interaction in cell immunoregulation. J Biomed Sci 2004;11:426-38.
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998;16:111-35.
- Zhang-Hoover J, Sutton A, Stein-Streilein J. CD40/CD40 ligand interactions are critical for elicitation of autoimmune-mediated fibrosis in the lung. J Immunol 2001;166:3556-63.
- Rossi G, Sarkar J, Scandella D. Long-term induction of immune tolerance after blockade of CD40-CD40L interaction in a mouse model of hemophilia A. Blood 2001;97:2750-7.
- Homann D, Jahreis A, Wolfe T, Hughes A, Coon B, van Stipdonk MJ, et al. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity 2002;16:403-15.
- Liu H, Mao N, Hou C, Li X, Shen P, Tang PX. Protective effect of human CD40-Ig fusion protein in a murine model of acute graft-versus-host disease. Chin Med J 2001;114:685-9.
- Wang X, Huang W, Mihara M, Sinha J, Davidson A. Mechanism of action of combined short-term CTLA4Ig and anti-CD40 ligand in murine systemic lupus erythematosus. J Immunol 2002;168:2046-53.
- Harbury PB, Zhang T, Kim PS, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science 1993;262:1401-7.
- Sorger PK, Nelson HC. Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell 1989;59:807-13.
- Harbury PB, Kim PS, Alber T. Crystal structure of an isoleucine-zipper trimer. Nature 1994;371:80-3.
- Morris AE, Remmele RL Jr, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154). J Biol Chem 1999;274:418-23.
- Shiraishi T, Suzuyama K, Okamoto H, Mineta T, Tabuchi K, Nakayama K, et al. Increased cytotoxicity of soluble Fas ligand by fusing isoleucine zipper motif. Biochem Biophys Res Commun 2004;322:197-202.
- Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci USA 2001;98:1751-6.
- He XH, Xu LH, Liu Y. Procedure for preparing peptide-major histocompatibility complex tetramers for direct quantification of antigen-specific cytotoxic T lymphocytes. World J Gastroenterol 2005;11:4180-7.
- Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA 1992;89:3429-33.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-5.
- Pietravalle F, Lecoanet-Henchoz S, Blasey H, Aubry JP, Elson G, Edgerton MD, et al. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J Biol Chem 1996;271:5965-7.
- Mazzei GJ, Edgerton MD, Losberger C, Lecoanet-Henchoz S, Graber P, Durandy A, et al. Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem 1995;270:7025-8.
- Contin C, Pitard V, Delmas Y, Pelletier N, Defrance T, Moreau JF, et al. Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology 2003;110:131-40.
- Holler N, Kataoka T, Bodmer JL, Romero P, Romero J, Deperthes D, et al. Development of improved soluble inhibitors of FasL and CD40L based on oligomerized receptors. J Immunol Methods 2000;237:159-73.
- Bajorath J, Aruffo A. Construction and analysis of a detailed three-dimensional model of the ligand binding domain of the human B cell receptor CD40. Proteins 1997;27:59-70.
- Singh J, Garber E, Van Vlijmen H, Karpusas M, Hsu YM, Zheng Z, et al. The role of polar interactions in the molecular recognition of CD40L with its receptor CD40. Protein Sci 1998;7:1124-35.
