Identification of human dopamine receptors agonists from Chinese herbs
Introduction
Dopamine receptors are known to play important roles in the regulation of cardiovascular function, renal function and systemic blood pressure[1]. Based on their structures and pharmacology[2–4], the receptors are divided into 2 subfa-milies: D1-like and D2-like. They all belong to the cell-surface G-protein coupled receptor (GPCR) super family. D1-like receptors, including receptor 1 (D1R) and receptor 5 (D5R), mainly couple to stimulatory G proteins (Gs). For D2-like receptors, which consist of receptor 2 (D2R), receptor 3 and receptor 4, they mainly link to inhibitory G proteins (Gi)[4]. The activation of D1-like receptors stimulates adenylyl cyclases (AC), increases the intracellular cAMP level which consequently activates cAMP responsive elements (CRE) and regulates the expression of target genes that are controlled byCRE[4–6]. As for D2-like receptors, the activation of receptors generate opposite effects since they link to the Gi protein and inhibit AC activity[4].
In the periphery system, dopamine receptors regulate blood pressure by altering renal hemodynamics, increasing renal sodium and water excretion via effects on renal proximal tubule epithelial cells, inhibiting sodium uptake in the gastrointestine, and releasing renin, aldosterone and vasopressin[7–9]. In the central systems, dopamine can act on the “appetite” center in the brain to regulate sodium and fluid intake[10].
Although all 5 dopamine receptor subtypes participate in the regulation of blood pressure, they seem to influence it by different mechanisms and various degrees. The activation of renal D1-like receptors has natriuretic and diuretic effects, mainly because D1-like receptors can inhibit Na,H-exchanger and Na,K-ATPase on the renal proximal tubular cell membrane by cAMP and cAMP-independent path-ways[6,11–14]. Na,H-exchanger and Na,K-ATPase are responsible for reabsorbing sodium and water from the renal proximal tubular lumina to the blood circulation. Thus, D1-like receptors can facilitate sodium and water excretion, resulting in lowering blood pressure[15,16]. Many reports have indicated that abnormalities in dopamine biosynthesis or defects in D1-like receptors may play an important role in the pathogenesis of hypertension[3,7,13,17,18]. The uncoupling of D1-like receptors from their G protein, possibly resulting from abnormalities and/or disabilities of GPCR kinases[19], may contribute to genetic hypertension[20–22]. D5R can inhibit the activity of phospholipase D2 (PLD2), and impaired D5R regulation of PLD2 may play a role in hypertension pathogenesis[23]. Furthermore, both D1R and D5R mutant mice developed hypertension[17,24]. On the contrary, the role of the D2-like receptor in the regulation of blood pressure is not yet well defined. All 3 D2-like receptors knockout mice were reported as hypertensive[25–27], and the reports indicate that the activation of D2-like receptors inhibit Na,H-exchanger[28]. Some other reports suggest that the activation of D2-like receptors produces antidiuresis and antinatriuresis[29,30].
Currently, there are several D1-like receptor agonists used as antihypertension drugs in the clinic, such as fenoldopam and dopexamine[31–33]. However, these 2 drugs are used only for hypertensive crises and in intensive care. Fenoldopam has poor bioavailability and may case allergic-type reactions, while dopexamine can activate multiple receptors and must be administered intravenously. The development of new antihypertension drugs remains an important task for the pharmaceutical industry. In China, many herbs have been used for the treatment of hypertension in the practice for many centuries. The natural compounds from these herbs could provide a rich source in the search for new candidates for antihypertension drugs. To explore this possibility, we used an in-house made, herb-based natural compound library[34] and developed cell-based dopamine receptor assays to perform high throughput screenings (HTS). In this article, we describe in detail the process of assay development for dopamine receptors, compound screening using these assays and hit compounds identification. Our work provides an alternative vision of how to use herb medicines and a way to develop antihypertension drugs.
Materials and methods
Plasmid construction The mouse G15 cDNA was cloned by RT-PCR of mRNA from murine blood cell, using primers 5'-CGCGGATCCACCATGGCCCGGTCCCTGACT-3' and 5'-CGCGGATCCTCACAGCAGGTTGATCTCGTCC-3', which were designed based on the G15 sequences (Gene Bank Accession N
Cell culture, transfection and clone selection The human embryonic kidney cell line, HEK-293, was cultured in a humidified 5% CO2 atmosphere at 37 oC in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, and 2 mmol/L L-glutamine. pcDNA1.1-4×CRE-blax/neo+ and pIRES/G15 were transfected to HEK-293 respec-tively, using Lipofectamine 2000 (Invitrogen,Carlsbad, CA, USA). The cells were selected by the addition of 0.7 mg/mL G418 in the culture medium for 10–14 d. The isolation of HEK/CRE-blax cell clones were performed by stimulation of cellular clones with forskolin (1 µmol/L). The clones, which turned to blue from green under fluorescence microscopy detection were individually isolated. The sequential transfection of plasmid containing human D1R or D5R genes was carried out to obtain HEK/D1R/CRE-blax and HEK/D5R/CRE-blax stable cell lines. The transfected cells were selected under a medium containing 0.2 mg/mL hygromycin. The selection of receptor positive clones was achieved by the stimulation of cells with 10 µmol/L dopamine in assay medium (serum-free Dulbecco’s Modified Eagle Medium (DMEM) with 0.1% bovine serum albumin). pIRES/G15 cell clones were identified by transiently transfecting a plasmid vector expressing Muscarinic M2 receptor (M2R). The clones with better calcium flux signal in the M2R activation assay (internal unpublished method) were selected as the parental cell line. The transfection and clone selection of D2R is the same as described for the D1-like receptor, except that the assays for the selection process were based on intracellular calcium flux.
Herbal extraction and natural compound library Sixty five herbal plants with therapeutic indications for hypertension treatment based on traditional Chinese medicine (TCM) were collected from a herb market in Anguo, Northern China. The herbs (100 g each, dry weight) were soaked in 90% ethanol for 12 h and extracted by ultrasonic for 40 min. The remains were extracted again with 50% ethanol for 12 h and by ultrasonic for 40 min. The extracted solvents were combined and filtered and lyophilized using a Freeze Dry System Plus 6 (Labconco, Kansas City, MO, USA). The lyophilized samples were resolved in ethanol and were subjected to HPLC fractionation using a Shimadzu 10A VP-HPLC system (Shimadzu Co, Kyoto, Japan). The fractionated samples (16 fractions for each extract) were lyophilized again, then re-dissolved in dimethyl sulphoxide (DMSO) and stored in 96-well sample plates at -80 oC for assays and screening.
β-lactamase assay high throughput screening The cells were seeded at 2x104cells/well, in black clear-bottom 96-well microplates (Corning Incorporated, Corning NY, USA). One d after, the medium was replaced by assay medium and then the cells were continually cultured overnight. On d 3, the compounds in the assay medium, along with the controls, were added into the plates for the assay. After 5 h stimulation, CCF4/AM (Invitrogen,Carlsbad, CA, USA) was loaded into the cells based on the protocol from the dye provider, and the cells were then incubated at room temperature for an additional 1–2 h in the dark[35]. The plates were read in the Analyst plate reader (Molecular Devices, Sunnyvale, CA, USA) with 405 nm excitation and 460 (blue fluorescence) and 530 nm (green fluorescence) emission[36]. The data was plotted as a ratio of the emissions at 460 and 530 nm. Two cell controls, 2 DMSO (1% or 0.5%) controls, 2 dopamine controls (positive) and 2 medium controls (background) were included in each plate for HTS. Hits that showed activity in the screening were identified, and a reconfirmation assay was performed for activity verification.
Fluorescent Ca2+-mobilization assay The cells were seeded into matrigel-coated, 96-well black clear-bottom plates at the density of 3×104cells/well. On d 2, 100 µL dye loading buffer from a HDB WASH Free Calcium Assay Kit (HD Biosciences, Shanghai, China) was added, and then the plates were incubated for 1 h at 37 oC. The assay plate was placed in the FlexStation II (Molecular Devices), followed by stimulation using 50 µL of 5×concentrated compounds, and real-time read at 485 nm excitation and 525 nm emission wavelengths to determine fluorescent responses.
Isolation and identification of hit compounds from herbs The herbs that possess activity (hit samples) were extracted with a larger scale and were HPLC fractionated as described earlier. The active fractions were further isolated until single peaks were achieved in an effort to obtain single compounds. The isolated compounds were subjected to activity verification using the HEK/D5R/CRE-blax assay cell line as well as parental control cells (HEK/CRE-blax), and the dose responsive curve determination using respective receptor assay cell lines.
Results
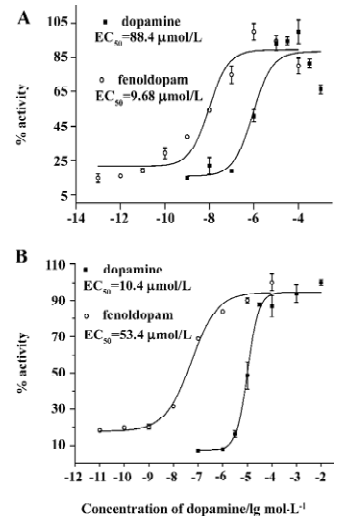
β-lactamase assay for D1R and D5R D1R and D5R are 2 Gs-coupled GPCR. We transfected 2 vectors carrying cDNA of D1R or D5R, to a parental cell line, HEK/CRE-blax. After antibiotics selection and dopamine screening, we obtained 2 cell lines, HEK/D1R/CRE-blax and HEK/D5R/CRE-blax, which express both receptor gene and reporter gene. Stimulated by dopamine or fenoldopam, a D1-like receptor agonist, the cells showed the expression of β-lactamase and the activity of the enzyme, which could be reflected by cleavage of CCF4 substrate and ratio change of fluorescence emission at 460 to 530 nm. The responses to agonist stimulation were in a dose-dependent manner (Figure 1A,B).
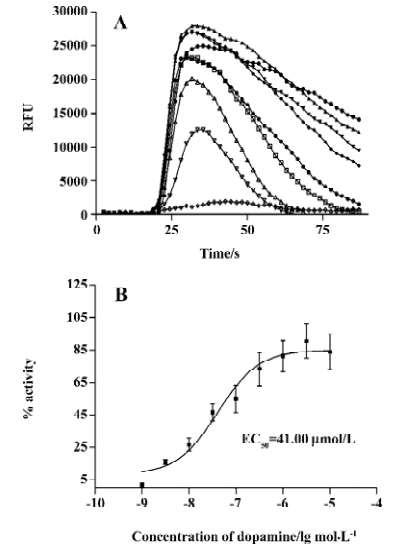
HEK/D2R/G15 cell line The D2R has been reported to couple to the Gi protein[4]. In order to perform a cell-based functional assay, we transfected the D2R gene into a HEK/G15 parental cell line and established a HEK/D2R/G15 cell line. With the selected cell line, we could value D2R activity by measuring intracellular Ca2+ mobilization. Figure 2 shows the result that dopamine can induce Ca2+ mobilization in HEK/D2R/G15 cell line (Figure 2A) and that the induction was in a dose-dependent manner with EC50 values of 41 nm (Figure 2B).
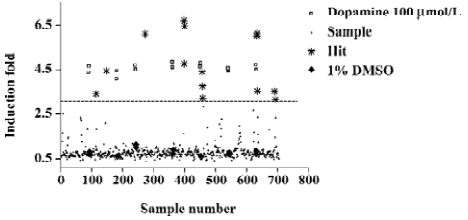
Screening for D1-like receptors agonists The HEK/D5R/CRE-blax was adopted to perform a HTS to identify dopamine receptor agonists from about 1000 HPLC fractionated samples from our herb extracts. Figure 3 shows an example of a data set from the screening of 700 diverse samples. As described earlier, the fold-change of 460 nm/530 nm ratio caused by cleavage of CCF4 substrate was used for hit compound identification. The Z’ values[37] from the screening ranged from 0.61 to 0.88 (>0.5 was considered acceptable). Fourteen samples were demonstrated with more than 5 folds of activation, and were retested for their activities using fresh dilution from the same DMSO compound stocks. A test using parental HEK/CRE-blax cells was also conducted to ensure that the activation of reporter was not due to the non-specific stimulation of signaling pathways by selected hit compounds.
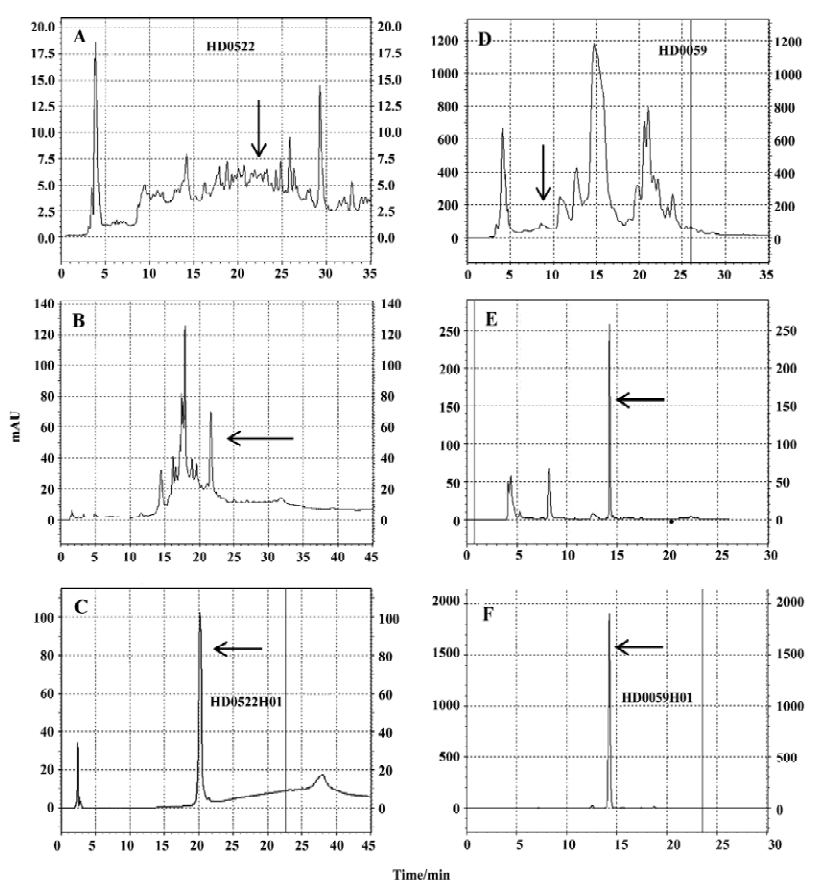
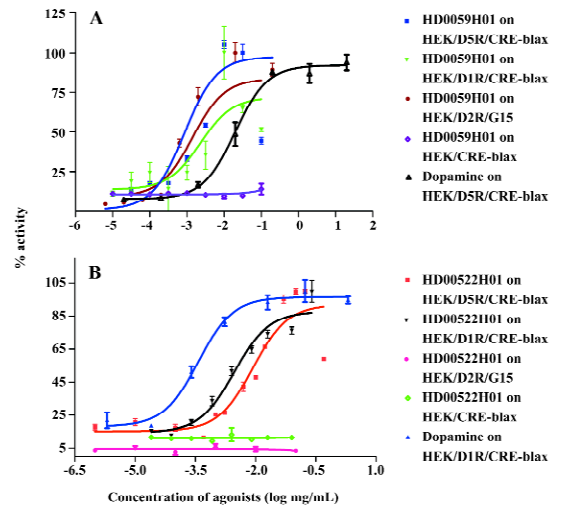
Identification of hit compounds Two herbs, HD0522 and HD0059, which generated fractionated samples and showed the better activities in the screening, were picked for further compound isolation and structure-activity relationship study. These active fractions were further purified by HPLC, and each single HPLC fraction was subsequently tested for its activity using the HEK/D5R/CRE-blax assay cell line. With this process, we finally obtained 2 single HPLC fractions (HD0522H01 and HD0059H01). Figure 4 shows HPLC isolation of HD0522H01 and HD0059H01. The assays for their activities toward D5R generated robust response (data not shown).
Hit compound specificity To find the possibility that HD0522H01 and HD0059H01 possess agonist specificity toward different dopamine receptors, we first examined their activities against 2 dopamine receptors (D1R, D2R, and D5R). Both samples demonstrated activities on both D1R and D5R (Figures 5, 6). The significant difference was observed in the test using D2R assay. While HD0059H01 could activate D2R in a dose-dependent manner with the EC50 of 0.85 µg/mL (Figure 5), HD0522H01 had no effect on the stimulation of HEK/D2R/G15 cells. The results suggest that HD0522H1 is an agonist specific to D1-like receptors, and HD059H01 is more like a non-specific agonist for general types of dopamine receptors. Another test is to find possible non-specific interactions between these 2 compounds with other GPCR. There was no stimulation effect between the 2 compounds and receptors that we tested (histamine H2, H4 receptors; mGluR2; data not shown). The results indicate that the 2 compounds are dopamine-specific agonists.
Discussion
Dopamine and renal dopamine receptors, especially D1-like receptors, play an important role in the regulation of sodium and body volume homeostasis. A defective renal dopaminergic system contributes to the development and maintenance of hypertension[1,3,4]. D1-like receptor agonists have been used in the therapy of clinic hypertension and relative diseases. D1-like receptors, D1R and D5R, are very similar in their structural and pharmacological characteristics and both couple efficiently to the Gs protein. In this study, we used the CRE element in conjugating with a reporter gene, β-lactamase, for the receptor activity assays, screening and the evaluation of natural agonist compounds. β-lactamase is a bacterial enzyme and has been widely used as a reporter gene to detect and quantify target gene expression in mammalian cells[35,38–40]. The β-lactamase reporter gene system is amenable for HTS because it utilizes a fluorometric readout on live cells so that cellular lysis or washes are not required to detect signals. An additional advantage of this β-lactamase reporter assay is that the detection is based on a self-quenched, fluorescence resonance energy transfer substrate, cell-permeable CCF4-AM. Upon stimulation by an agonist, D1-like receptor-expressing reporter cells show an induction of β-lactamase activity in our reporter gene system. The production of β-lactamase in this reporter cell line, the subsequent cleavage of the CCF4 substrate, and the generation of blue fluorescent cells can be attributed to the activation of signal transduction, initiated by the activation of D1-like receptors. The change in color of fluorescence provides a simple and sensitive way of visualizing whether the expression of β-lactamase is activated, and whether a cell population is homogenous. This receptor-reporter gene assay has been optimized into a sensitive, robust, high-throughput screening assay executed in a 96-well microplate format.
Using the HTS assay system, we obtained 14 active hits against D5R. The test of these hits using the HEK/CRE-blax parental cell line generates negative reporter gene response, demonstrating that these hit compounds have no self-fluorescence and are all receptor specific. The 2 best hits, HD0522H01 and HD0059H01, were purified using HPLC in the highest possibility in our current experiment condition. The purity was reconfirmed by a change of elution condition and columns. It has been a common phenomenon that the purification of single compounds from natural products results in the loss of activity[41]. Our work provides an example for successfully tracing possible single active components from extracts of herbal plants. In a separate test, both HD0522H01 and HD0059H01 exhibited no cross activities with several other GPCR in the same type of cell-based assays, suggesting that they are not universal agonists that are commonly seen in many HTS hit identification processes.
To study these 2 hits’ specificities among different dopamine receptor subtypes, we established a D2R assay cell line, HEK/D2R/G15. The D2R belongs to a Gi-coupled receptor group. In their native environment, the activation of receptors can not produce significant intracellular calcium flux for the assays. The promiscuous G proteins, G15 and G16, belong to the Gq protein family, but they can couple to a variety of GPCR, not only Gq-coupled GPCR, but also Gi- and Gs-coupled GPCR[42,43]. Our study shows that D2R couples to G15, resulting in the activation of the IP3 pathway and increase of cellular calcium concentration, so we used the G15 protein in D2R screening by measuring the changes of intracellular calcium concentration.
One of the important findings in this work is that HD0059H01 could activate all 3 dopamine receptors (D1R, D5R and D2R), in a dose-dependent manner, but HD0522H01 possessed receptor specificity. The later hit could only activate D1-like receptors. Based on current understandings of the relationship between dopamine receptors and hypertension, it seems that HD0522H01 is a better candidate as an antihypertension drug. On the other hand, HD0059H01 also originated from a herb with antihypertension indication. The results may provide evidence that the agonists without the high specificity could also be used for hypertension treatment. Furthermore, the studies we conducted and the results from the studies indicate the possible mechanisms of those herbs in their effects on the treatment of hypertension.
Taken together, our study has identified 2 dopamine receptor agonists, HD0522H01 and HD0059H01, from herb-based natural products. One of them, HD0522H01, is a D1-like receptor specific. The hit compounds could be good candidates as potential antihypertension drug leads. The specific hit, HD0522H01, might be more promising because the D1-like receptors seem to be more closely related to hypertension, the treatment of hypertension, and are less likely to cause side effects.
Acknowledgements
The authors would like to thank Hong-ru LIU, Ya-qin JIANG and Peng CUI for their excellent technical assistance.
References
- Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther 1998;80:149-82.
- Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol 2003;95:19-27.
- Jose PA, Eisner GM, Drago J, Carey RM, Felder RA. Dopamine receptor signaling defects in spontaneous hypertension. Am J Hypertens 1996;9:400-5.
- Sibley DR. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu Rev Pharmacol Toxicol 1999;39:313-41.
- Uh M, White BH, Sidhu A. Alteration of association of agonist-activated renal D1(A) dopamine receptors with G proteins in proximal tubules of the spontaneously hypertensive rat. J Hypertens 1998;16:1307-13.
- Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, et al. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol 2000;278:R1064-73.
- van den Buuse M. Role of the mesolimbic dopamine system in cardiovascular homeostasis. Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin Exp Pharmacol Physiol 1998;25:661-8.
- Sowers JR, Viosca SP, Windsor C, Korenman SG. Influence of dopaminergic mechanisms on 24-hour secretory patterns of prolactin, luteinizing hormone and testosterone in recumbent men. J Endocrinol Invest 1983;6:9-15.
- Vieira-Coelho MA, Teixeira VA, Finkel Y, Soares-Da-Silva P, Bertorello AM. Dopamine-dependent inhibition of jejunal Na+-K+-ATPase during high-salt diet in young but not in adult rats. Am J Physiol 1998;275:G1317-23.
- Roitman MF, Schafe GE, Thiele TE, Bernstein IL. Dopamine and sodium appetite: antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci 1997;111:606-11.
- Baines AD, Drangova R. Does dopamine use several signal pathways to inhibit Na-Pi transport in OK cells? J Am Soc Nephrol 1998;9:1604-12.
- Feschenko MS, Stevenson E, Sweadner KJ. Interaction of protein kinase C and cAMP-dependent pathways in the phosphorylation of the Na,K-ATPase. J Biol Chem 2000;275:34693-700.
- Hussain T, Lokhandwala MF. Dopamine-1 receptor G-protein coupling and the involvement of phospholipase A2 in dopamine-1 receptor mediated cellular signaling mechanisms in the proximal tubules of SHR. Clin Exp Hypertens 1997;19:131-40.
- Lin F, Abraham NG, Schwartzman ML. Cytochrome P450 arachidonic acid omega-hydroxylation in the proximal tubule of the rat kidney. Ann N Y Acad Sci 1994;744:11-24.
- Li XX, Albrecht FE, Robillard JE, Eisner GM, Jose PA. Gbeta regulation of Na/H exchanger-3 activity in rat renal proximal tubules during development. Am J Physiol Regul Integr Comp Physiol 2000;278:R931-6.
- Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Feraille E, et al. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J Biol Chem 1999;274:1920-7.
- Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, et al. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest 1996;97:2283-8.
- de Vries PA, Navis G, de Jong PE, de Zeeuw D, Kluppel CA. Impaired renal vascular response to a D1-like receptor agonist but not to an ACE inhibitor in conscious spontaneously hypertensive rats. J Cardiovasc Pharmacol 1999;34:191-8.
- Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA 2002;99:3872-7.
- Hussain T, Lokhandwala MF. Altered arachidonic acid metabolism contributes to the failure of dopamine to inhibit Na+,K(+)-ATPase in kidney of spontaneously hypertensive rats. Clin Exp Hypertens 1996;18:963-74.
- Debska-Slizien A, Ho P, Drangova R, Baines AD. Endogenous dopamine regulates phosphate reabsorption but not NaK-ATPase in spontaneously hypertensive rat kidneys. J Am Soc Nephrol 1994;5:1125-32.
- Chatziantoniou C, Ruan X, Arendshorst WJ. Defective G protein activation of the cAMP pathway in rat kidney during genetic hypertension. Proc Natl Acad Sci USA 1995;92:2924-8.
- Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Bai RK, et al. D5 dopamine receptor regulation of phospholipase D. Am J Physiol Heart Circ Physiol 2005;288:H55-61.
- Yang Z, Sibley DR, Jose PA. D5 dopamine receptor knockout mice and hypertension. J Recept Signal Transduct Res 2004;24:149-64.
- Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, et al. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest 1998;102:493-8.
- Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, et al. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 2006;47:288-95.
- Li XX, Bek M, Asico LD, Yang ZW, Rubinstein M, Grandy DK, et al. Disruption of the D(2) dopamine receptor produces adrenergic and endothelin B receptor-dependent hypertension. Zhonghua Yi Xue Za Zhi 2002;82:703-7. Chinese..
- Pedrosa R, Gomes P, Zeng C, Hopfer U, Jose PA, Soares-da-Silva P. Dopamine D3 receptor-mediated inhibition of Na+/H+ exchanger activity in normotensive and spontaneously hypertensive rat proximal tubular epithelial cells. Br J Pharmacol 2004;142:1343-53.
- Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Evidence that dopamine-2 mechanisms control renal function. Am J Physiol 1990;259:F793-800.
- Siragy HM, Felder RA, Peach MJ, Carey RM. Intrarenal DA2 dopamine receptor stimulation in the conscious dog. Am J Physiol 1992;262:F932-8.
- Oparil S, Aronson S, Deeb GM, Epstein M, Levy JH, Luther RR, et al. Fenoldopam: a new parenteral antihypertensive: consensus roundtable on the management of perioperative hypertension and hypertensive crises. Am J Hypertens 1999;12:653-64.
- Colvin JR, Chaudhri S, Kenny GN. Evaluation of dopexamine hydrochloride as an anti-hypertensive agent after cardiac surgery. Int J Clin Monit Comput 1991;8:95-100.
- Ghosh S, Gray B, Oduro A, Latimer RD. Dopexamine hydrochlo-ride: pharmacology and use in low cardiac output states. J Cardio-thorac Vasc Anesth 1991;5:382-9.
- Bindseil KU, Jakupovic J, Wolf D, Lavayre J, Leboul J, van der Pyl D. Pure compound libraries; a new perspective for natural product based drug discovery. Drug Discov Today 2001;6:840-7.
- Zlokarnik G. Fusions to beta-lactamase as a reporter for gene expression in live mammalian cells. Methods Enzymol 2000;326:221-44.
- Kunapuli P, Ransom R, Murphy KL, Pettibone D, Kerby J, Grimwood S, et al. Development of an intact cell reporter gene beta-lactamase assay for G protein-coupled receptors for high-throughput screening. Anal Biochem 2003;314:16-29.
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 1999;4:67-73.
- Zlokarnik G, Negulescu PA, Knapp TE, Mere L, Burres N, Feng L, et al. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science 1998;279:84-8.
- Peekhaus NT, Ferrer M, Chang T, Kornienko O, Schneeweis JE, Smith TS, et al. A beta-lactamase-dependent Gal4-estrogen receptor beta transactivation assay for the ultra-high throughput screening of estrogen receptor beta agonists in a 3456-well format. Assay Drug Dev Technol 2003;1:789-800.
- Zuck P, Murray EM, Stec E, Grobler JA, Simon AJ, Strulovici B, et al. A cell-based beta-lactamase reporter gene assay for the identification of inhibitors of hepatitis C virus replication. Anal Biochem 2004;334:344-55.
- Rishton GM. Failure and success in modern drug discovery: guiding principles in the establishment of high probability of success drug discovery organizations. Med Chem 2005;1:519-27.
- Offermanns S, Simon MI. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem 1995;270:15175-80.
- Liu AM, Ho MK, Wong CS, Chan JH, Pau AH, Wong YH. Galpha(16/z) chimeras efficiently link a wide range of G protein-coupled receptors to calcium mobilization. J Biomol Screen 2003;8:39-49.
