Serum amyloid A induces WISH cell apoptosis1
Introduction
Serum amyloid A (SAA) is an important acute reactant with wide-ranging pathological activities[1,2]. SAA has been reported to induce chemotactic migration in monocytes and neutrophils[3,4]. Some reports have demonstrated that SAA affects cytokine production in several cell types, such as neutrophils and monocytes[5,6]. In terms of SAA cell surface receptors, a member of G-protein coupled receptors called formyl peptide receptor like-1 (FPRL1) has been reported[7]. Although many previous reports have demonstrated the pivotal role of SAA in the modulation of several biological responses, the expression of the SAA receptor in amnion cells, and the role of SAA in these cells, have not been previously studied.
SAA can be dramatically produced against infected conditions[1,2]. Hepatocyte is regarded as a major cell for SAA synthesis, and SAA can be generated by various inflammatory stimuli, including tumor necrosis factor-α[8]. Previously, SAA has been shown to be present in both maternal and fetal plasma in the period around parturition[9]. It has also been reported that ovine allantoic fluid contains high concentrations of SAA-like protein[10]. However, the role of SAA in the regulation of reproduction has not been fully investi-gated.
In this study, we aim to investigate whether the SAA receptor is expressed on human amnion-derived WISH cells and if SAA modulates cellular activity of cells. We also investigated the signaling pathways involved in the SAA-mediated regulation of WISH cell activity.
Materials and methods
Reagents RPMI-1640 medium, dialyzed fetal bovine serum (FBS), and the RT-PCR kit were purchased from Invitrogen Corp (Carlsbad, CA, USA). Enhanced chemiluminescence reagents were from GE Healthcare Bio-Sciences Corp (Piscataway, NJ, USA), and phospho- extracellular signal-regulated protein kinase (ERK) 1/2, phospho-p38 kinase, and ERK2 antibodies were from New England Biolabs (Beverly, MA, USA). Rabbit polyclonal anti-caspase-3 antibodies were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Pertussis toxin (PTX), 2'-amino-3'-methoxyflav-one (PD98059), 4-(4-fluorophenyl)-2-(4-methylsulfinyl-phenyl)-5-(4-pyridyl)1H-imidazole (SB203580), and 1-{6-[(17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino]hexyl}-1H-pyrrole-2,5-dione (U-73122) were obtained from Calbiochem (San Diego, CA, USA). PD98059, SB203580 and U-73122 were dissolved in dimethyl sulfoxide before being added to cell culture. The final concentrations of dimethyl sulfoxide in culture were 0.1% or less.
Cell culture The WISH human amnion cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). The WISH cells were cultured in RPMI-1640 media supplemented with 2 µmol/L L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% FBS at 37 °C in a humidified 5% CO2 atmosphere. The cells were subcultured twice weekly by trypsinization and seeded in 6-well plates (5×105 cells/well).
RT-PCR analysis mRNA was isolated using a QIAshredder and an RNeasy kit (Qiagen, Hilden, Germany). mRNA, M-MLV RT, and pd(N)6 primers (Invitrogen, USA) were used to obtain cDNA. The primers used for the RT-PCR analysis have been reported previously[11]. The sequences of the primer used were as follows; FPR: forward, 5'-CTCCAGTT-GGACTAGCCACA-3'; reverse, 5'-CCATCACCCAGGGCCC-AATG-3'; FPRL1: forward, 5'-CTGCTGGTGCT-GCTGGCAAG-3'; reverse, 5'-AATATCCCTGACCCCATCCTCA-3'; FPRL2: forward, 5'-GCCAAGGTCTTTCTGATCC-3'; reverse, 5'- GGTCTGGGCTGAGTCAGGGA-3'. We ran 30 PCR cycles of 94 °C (denaturation, 1 min), 59 °C (annealing, 1 min) and 72 °C (extension, 1 min). PCR products were electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining.
Ligand binding assay Ligand binding assay was performed as described previously[12]. Radioiodinated Trp-Lys-Tyr-Met-Val-D-Met (WKYMVm) (125I-labeled) was purchased from PerkinElmer Life and Analytical Sciences Inc (Wellesley, MA, USA). Briefly, the WISH cells were seeded at 1×105 cells per well into a 24-well plate and cultured overnight. After treating the cells with blocking buffer [33 mmol/L HEPES, pH 7.5, 0.1% bovine serum albumin (BSA) in RPMI- 1640 medium] for 2 h, 50 pmol/L of labeled WKYMVm was added to the cells in binding buffer [phosphate buffered saline (PBS) containing 0.1% BSA] in the absence or presence of unlabelled WKYMVm. The mixture was then incubated for 3 h at 4 °C with continuous agitation. The samples were then washed 5 times with ice-cold binding buffer, and 200 µL of lysis buffer (20 mmol/L Tris, pH 7.5, 1% Triton X-100) was added to each well. After incubation for 20 min at room temperature, the lysates were collected and the associated radioactivity was determined using a γ-ray counter[12].
Cellular proliferation assay For SAA treatment, the cells were seeded in 24-well plates, in triplicate, at 5×104 cells/well. Complete medium was replaced with serum-free RPMI-1640 medium to starve the cells for 24 h. SAA was added to the cultures to promote growth activity with or without several kinase inhibitors. [3H]Thymidine (1 µCi/mL) was added and incubation continued for 24 h. The medium was then removed and the cells were fixed with 5% cold trichloroacetic acid (TCA) for 15 min. TCA-precipitated material was solubilized in 1 mol/L NaOH for 1 h and neutralized by adding 1 mol/L HCl. [3H]Thymidine uptake was determined by liquid scintillation counting.
Nuclear staining with 4,6-diamino-2-phenylindole (DAPI) The cells were washed with PBS, fixed with cold methanol for 2 min at room temperature, washed with PBS, and stained with DAPI solution (Sigma, USA) for 10 min at room temperature. They were then washed 2 more times with PBS and analyzed under a fluorescence microscope[13].
Western blot analysis The WISH cells were plated in a 6-well plate and treated with SAA at different times. The cells were then washed with cold-PBS, scraped off, and pelleted at 700×g at 4 °C. The cell pellet obtained was resuspended in lysis buffer (20 mmol/L HEPES, pH 7.2, 10% glycerol, 150 mmol/L NaCl, 1% Triton X-100, 50 mmol/L NaF, 1 mmol/L Na3VO4, 10 µg/mL leupeptin, 10 mg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, and protease inhibitor cocktail), cleared by centrifugation, and the supernatant saved as a whole-cell lysate. Proteins (30 µg) were separated by 10% reducing SDS-PAGE and electroblotted in 20% methanol, 25 mmol/L Tris and 192 mmol/L glycine onto a nitrocellulose membrane. The membrane was then blocked with 5% nonfat dry milk in Tris-buffered saline-Tween 20 (25 mmol/L Tris-HCl, 150 mmol/L NaCl and 0.2% Tween 20), incubated with antibodies for 4 h, washed, and re-incubated for 1 h with secondary antibodies conjugated to horseradish peroxidase. Finally, the membrane was washed and developed using an Enhanced Chemiluminescence (ECL) system.
Statistical analysis The results are expressed as mean±SEM of the number of determinations indicated. Statistical significance of differences was determined by ANOVA. Significance was accepted when P<0.05.
Results
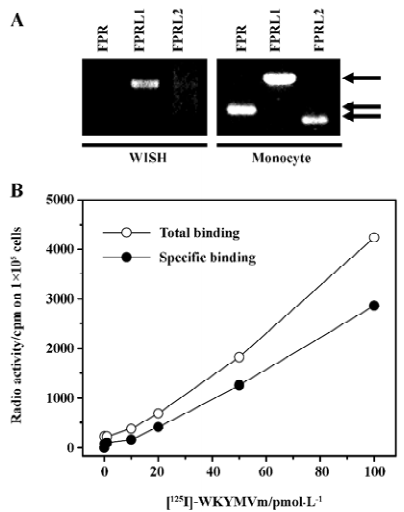
Expression of the SAA receptor in WISH cells We investigated whether the cell surface receptor for SAA is expressed on WISH cells. To determine whether the SAA receptor is expressed on WISH cells, we analyzed the mRNA expressions of FPRL1 by semiquantitative RT-PCR. As shown in Figure 1A, WISH cells express SAA receptor, namely, FPRL1. However, WISH cells do not express the other 2 FPR family receptors, FPR and FPRL2 (Figure 1A). We confirmed that the RT-PCR product obtained without the addition of RT did not contain a DNA band (data not shown). We also determined whether FPRL1 is expressed on WISH cells by using ligand binding assay with [125I]WKYMVm, a ligand for FPRL1. As shown in Figure 1B, the addition of various concentrations of [125I]WKYMVm demonstrated the concentration-dependent binding of [125I]WKYMVm to WISH cells (Figure 1B), which was quantified after subtracting nonspecific binding. The addition of molar excess of unlabeled WKYMVm (10 µmol/L) prior to the addition of [125I]WKYMVm reduced this binding. The specific binding of WKYMVm in WISH cells was proven by adding 25–100 pmol/L of [125I]WKYMVm for 5×104 cells (Figure 1B). This result indicates that WISH cells express the functional SAA receptor, FPRL1.
SAA decreased proliferating cell population, resulting in apoptosis in WISH cells In order to examine the effect of SAA on the proliferating cell population, we investigated the effect of SAA on [3H]thymidine incorporation in WISH cells. As shown in Figure 2A, the stimulation of WISH cells by several concentrations of SAA for 24 h inhibited [3H]thymidine incorporation in a concentration-dependent manner. The stimulation of WISH cells with 2 µmol/L of SAA inhibited [3H]thymidine incorporation by around half versus the unstimulated control (Figure 2A). To determine whether the growth inhibitory effect of SAA is associated with apoptosis, we examined WISH cell morphological changes after treatment with SAA. WISH cells growing on culture plates were exposed to 2 µmol/L SAA for 24 h. As shown in Figure 2B, SAA caused significant levels of cell shrinkage and chromatin condensation, thus showing that SAA induces WISH cell apoptosis. As a positive control, hydrogen peroxide (2 mmol/L) also caused WISH cell apoptosis (Figure 2B).
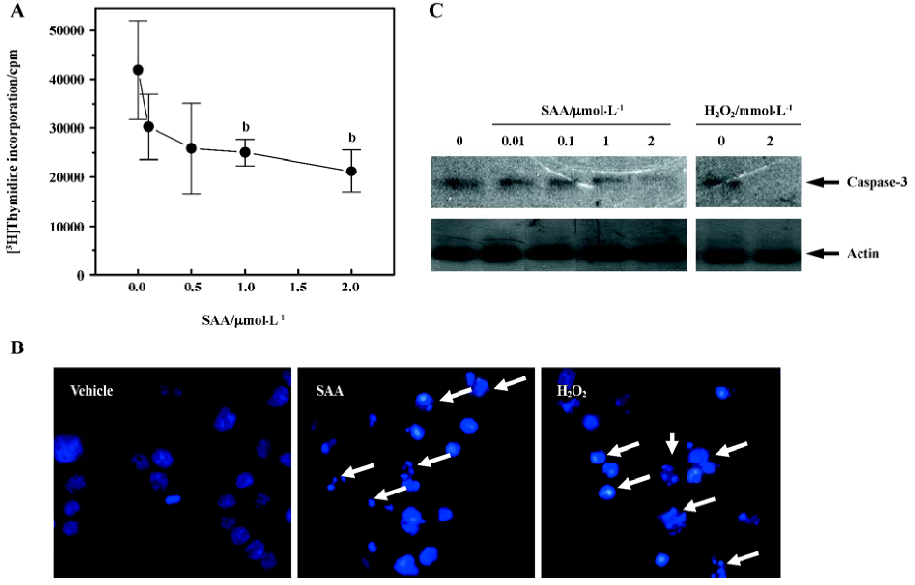
Caspase is a member of a family of proteases that are involved in proteolytic cleavage of cellular proteins during apoptosis[14]. Many reports have shown that apoptosis is accompanied by the activation of caspases, especially caspase-3[14–16]. We studied the effect of SAA on caspase-3 activity. Since caspase-3 is known to be cleaved during its activation process[14,15], we confirmed the effect of SAA on caspase-3 activity by Western blot analysis using an antibody that recognizes caspase-3. Treatment with various concentrations of SAA induced the cleavage of caspase-3, a mark for its activation, in a concentration-dependent manner (Figure 2C). These results coincide with the inhibitory effect of SAA on thymidine incorporation in WISH cells (Figure 2A).
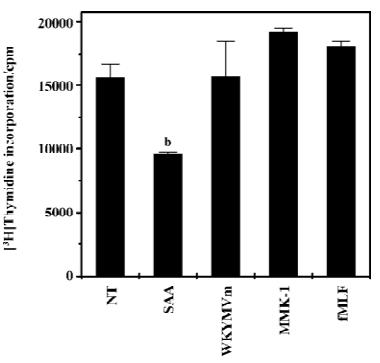
Among FPRL1 agonists, SAA selectively decreased proliferating cell population in WISH cells Previous reports have demonstrated that SAA binds to FPRL1, an important classical chemoattractant receptor[4,7]. Since in this study SAA decreased proliferating cell population, we also investigated the effect of several FPRL1 agonists on the proliferating cell population in WISH cells. As shown in Figure 3, among the tested FPRL1 agonists, only SAA showed a decrease of proliferating cell population in WISH cells. The other FPRL1 agonists such as WKYMVm[17,18] and Leu-Glu-Ser-Ile-Phe-Arg-Ser-Leu-Leu-Phe-Arg-Val-Met (LESIFRSL-LFRVM, MMK-1)[19,20] had no effect on the proliferating cell population in WISH cells (Figure 3), suggesting a SAA-selective response. FPR-selective agonist, formyl-Met-Leu-Phe (fMLF) also did not affect the proliferating cell population in WISH cells (Figure 3).
SAA stimulates mitogen-activated protein kinase in a pertussis toxin-insensitive manner in WISH cells Mitogen-activated protein kinase (MAPK) has been reported to mediate extracellular signals to the nucleus in various cell types[21]. In this study, we examined whether SAA stimulates MAPK by Western blotting with antiphospho-specific antibodies to each enzyme. When the WISH cells were stimulated with 2 µmol/L SAA for different times, the phosphorylation level of ERK transiently increased, showing maximal activity after 2–5 min of stimulation (Figure 4A), and returning to the baseline 30 min after stimulation (Figure 4A). Another important MAPK, p38 kinase, was also transiently phosphorylated by SAA stimulation with kinetics that resembled those of ERK phosphorylation (Figure 4A).
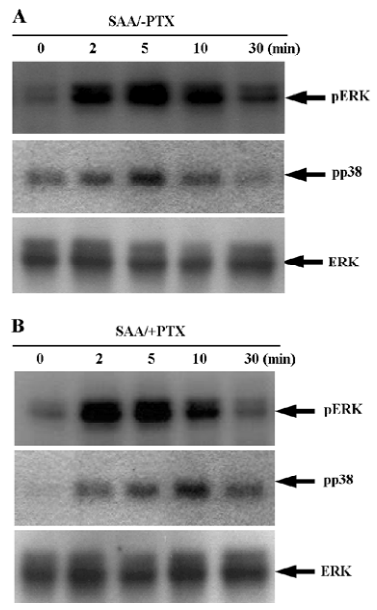
We investigated the role of pertussis toxin (PTX)-sensitive G-protein on SAA-induced ERK phosphorylation. Cultured WISH cells were pre-incubated with 100 ng/mL of PTX prior to being stimulated with 2 µmol/L SAA. We found that PTX pretreatment did not block ERK phosphorylation by SAA (Figure 4B), showing that SAA induces ERK phosphorylation in a PTX-insensitive manner. We also examined the effect of PTX on SAA-induced p38 kinase phosphoryla-tion. When the WISH cells were pre-incubated with 100 ng/mL of PTX prior to being stimulated with 2 µmol/L SAA, SAA-induced p38 kinase phosphorylation was not found to be blocked (Figure 4B). These results indicate that SAA stimulates ERK and p38 kinase phosphorylation via a PTX-sensitive G-protein-independent pathway.
Regulation of SAA-induced decrease of proliferating cell population Previously, SAA has been reported to act on cell surface receptor FPRL1, which is mainly coupled to PTX-sensitive Gi proteins[7]. We examined the effect of PTX on the SAA-decreased proliferating cell population in WISH cells. Pre-incubation of WISH cells with 100 ng/mL of PTX for 24 h dramatically inhibited sphingosine-1-phosphate-induced intracellular calcium increase (data not shown), suggesting that PTX completely inhibits Gi-mediated signaling in cells. In the same condition, pretreatment of PTX prior to the addition of SAA for 24 h did not affect the inhibitory activity on the decrease of the proliferating cell population in WISH cells (Figure 5A). The result indicates that SAA decreased the proliferating cell population in a PTX-insensitive manner.
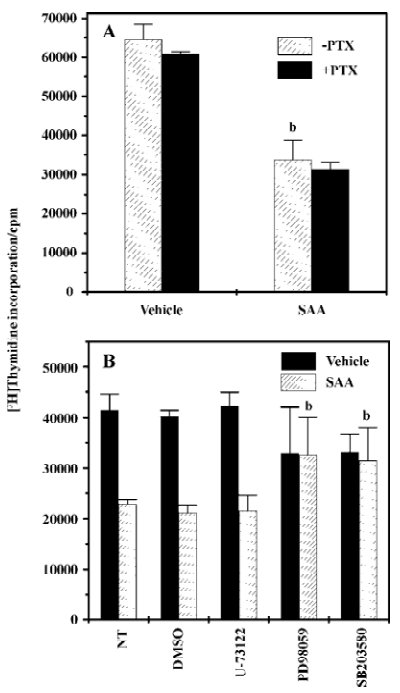
Many studies have demonstrated that p38 kinase is involved in cell proliferation inhibition[22,23]. In this study, we examined the effect of MAPK (ERK and p38 kinase) on SAA-decreased proliferating cell population in WISH cells. As shown in Figure 5B, SAA-inhibited [3H]thymidine incorporation was almost totally inhibited in the presence of PD98059 or in the presence of SB203580. These results suggest that ERK and p38 kinase are essential for SAA-inhibited [3H]thymidine incorporation by WISH cells. However, pre-incubation of WISH cells with 5 μmol/L of U-73122 did not affect the inhibitory effect of SAA on the decrease of the proliferating cell population in WISH cells (Figure 5B), suggesting that phospholipase C is not involved in the SAA-decreased proliferating cell population in WISH cells.
Discussion
Although many reports have shown that SAA is highly upregulated during infection, its role in amniotic cells has not been investigated. In the present study, we found that human amnion-derived WISH cells express SAA receptor FPRL1, which indicates that SAA may modulate the cellular activity of WISH cells. To identify the functional role of SAA stimulation in WISH cells, we focused on apoptosis. As shown in Figure 2, SAA potently induced apoptosis in WISH cells. Since amnion cell proliferation is important for the maintenance of pregnancy, these data in WISH cells suggests that SAA plays a negative role in the maintenance of pregnancy.
A previous report demonstrated that SAA binds to one of the G-protein coupled cell surface receptor, namely FPRL1[7]. When we performed RT-PCR to examine the expression pattern of the SAA receptor on WISH cells, we found that WISH cells express FPRL1 (Figure 1). According to previous reports, FPRL1 interact with G-proteins of the Gi families[24,25]. In our study, we investigated the effect of PTX, (which specifically inactivates Gi-mediated signaling pathways) on SAA-induced signaling. When WISH cells were pretreated with 100 ng/mL of PTX for 24 h prior to SAA stimulation, SAA-decreased proliferating cell population was not affected (Figure 5A), and SAA-stimulated MAPK (ERK and p38 kinase) activation was not blocked by PTX pretreatment (Figure 4). These results suggest that SAA modulates MAPK activation and leads to a decrease of the proliferating cell population, and that PTX-sensitive G-proteins are not involved in this process in WISH cells. The signaling pathway of the FPR family, including FPRL1, has been extensively studied[24,26]. Among the previous reports, Tsu et al, reported that FPR can also couple to Gi1, Go, and a PTX-insensitive G-protein, Gz, as shown by cotransfection experiments in HEK293 cells. They also demonstrated that in such conditions, Gz can only replace Gi in inhibiting cAMP accumulation, but not in stimulating phospholipase C (PLC)[27]. Keeping in mind that SAA binds to FPRL1 and the fact that SAA decreased proliferating cell population in a PTX-resistant manner, it suggests that SAA may stimulate PTX-insensitive signaling downstream of FPRL1.
To investigate the signal pathway of the effect of the decrease of the proliferating cell population by SAA in WISH cells, we examined the role of MAPK. We observed that SAA stimulated both ERK and p38 kinase activity (Figure 4A). To determine the role of ERK or p38 kinase on the SAA-decreased proliferating cell population, we pretreated WISH cells with 2 different MAPK-selective inhibitors, PD98059 and SB203580 [selective MAPK-kinase (MEK) and p38 kinase inhibitor, respectively]. Since we found that high concentrations of PD98059 (50 µmol/L) and SB203580 (20 µmol/L) affected thymidine incorporation by themselves, we used 10 μmol/L of PD98059 and 5 µmol/L of SB203580. The pre-incubation of WISH cells with PD98059 (10 µmol/L) or SB203580 (5 µmol/L) prior to SAA stimulation blocked the effect on the decrease of the proliferating cell population by SAA (Figure 5B). We also observed that PD98059 (10 µmol/L) and SB203580 (5 µmol/L) blocked SAA-induced MAPK activation (data not shown).This indicates that ERK and p38 kinase play key roles in the SAA-decreased proliferating cell population in amniotic cells. Many previous reports have demonstrated that p38 kinase is essentially required for the induction of cell death in several cell types[22,23]. However, p38 kinase also has been reported to inhibit cell death in certain cell types[28]. In the case of ERK, it also acts as a positive and negative mediator of cell death[29,30]. In our study, we demonstrated that ERK pathway inhibitor, PD98059, strongly blocked the decrease of the proliferating cell population by SAA in WISH cells (Figure 5B). It suggests that ERK is essential for the induction of the SAA-decreased proliferating cell population.
The SAA we used is a recombinant protein SAA which is produced in Escherichia coli. Even though the endotoxin content of SAA preparation is negligible (0.1 ng/mg), we further examined the possible contribution of lipopolysaccharide (LPS) on the SAA-induced decrease of the proliferating cell population using a potent inhibitor of LPS (polymyxin B). Pre-incubation of WISH cells with polymyxin B (10 µg/mL) prior to the addition of LPS completely inhibited ERK phosphorylation by LPS, but SAA-induced ERK phosphorylation and the decrease of proliferating cell population were unaffected (data not shown). From the results, we can rule out the possibility of an indirect decrease of the proliferating cell population by SAA, supporting our notion that SAA directly decreases the proliferating cell population in WISH cells via its specific cell surface receptor which is different from FPRL1.
In conclusion, the present study indicates that SAA acts on the SAA-specific receptor FPRL1, resulting in apoptosis in WISH cells which are widely used as a model system for amniotic epithelial cells. However, Kniss et al showed that the WISH cell line has been contaminated by Hela cells (a human cervical adenocarcinoma cell line)[31]. Since this study is the first to report on the expression of the SAA receptor on WISH cells, further studies on the pathophysiological and physiological roles of SAA in the maintenance of pregnancy response of primary cultures of human amniotic epithelial cells are required.
References
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 1999;265:501-23.
- Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J 1998;334:489-503.
- Badolato R, Johnston JA, Wang JM, McVicar D, Xu LL, Oppenheim JJ, et al. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J Immunol 1995;155:4004-10.
- Liang TS, Wang JM, Murphy PM, Gao JL. Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem Biophys Res Commun 2000;270:331-5.
- He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 2003;10:1572-81.
- Patel H, Fellowes R, Coade S, Woo P. Human serum amyloid A has cytokine-like properties. Scand J Immunol 1998;48:410-8.
- Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med 1999;189:395-402.
- Lowell CA, Stearman RS, Morrow JF. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem 1986;261:8453-61.
- Alsemgeest SP, Taverne MA, Boosman R, van der Weyden BC, Gruys E. Peripartum acute-phase protein serum amyloid-A concentration in plasma of cows and fetuses. Am J Vet Res 1993;54:164-7.
- Foulds LM, de Kretser DM, Farnworth P, Buttress D, Jenkin G, Groome NP, et al. Ovine allantoic fluid contains high concentrations of activin A: partial dissociation of immunoactivity and bioactivity. Biol Reprod 1998;59:233-40.
- Kang HK, Lee HY, Kim MK, Park KS, Park YM, Kwak JY, et al. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met inhibits human monocyte-derived dendritic cell maturation via formyl peptide receptor and formyl peptide receptor-like 2. J Immunol 2005;175:685-92.
- Bae YS, Yi HJ, Lee HY, Jo EJ, Kim JI, Lee TG, et al. Differential activation of formyl peptide receptor-like 1 by peptide ligands. J Immunol 2003;171:6807-13.
- Lee YN, Lee HY, Kim JS, Park C, Choi YH, Lee TG, et al. The novel phospholipase C activator, m-3M3FBS, induces monocytic leukemia cell apoptosis. Cancer Lett 2005;222:227-35.
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol 2003;15:725-31.
- Kim JM, Bae HR, Park BS, Lee JM, Ahn HB, Rho JH, et al. Early mitochondrial hyperpolarization and intracellular alkalinization in lactacystin-induced apoptosis of retinal pigment epithelial cells. J Pharmacol Exp Ther 2003;305:474-81.
- Lee SW, Lee HJ, Chung WT, Choi SM, Rhyu SH, Kim DK, et al. TRAIL induces apoptosis of chondrocytes and influences the pathogenesis of experimentally induced rat osteoarthritis. Arthritis Rheum 2004;50:534-42.
- Seo JK, Choi SY, Kim Y, Baek SH, Kim KY, Chae CB, et al. A peptide with unique receptor specificity: stimulation of phospho-inositide hydrolysis and induction of superoxide generation in human neutrophils. J Immunol 1997;158:1895-901.
- Le Y, Gong W, Li B, Dunlop NM, Shen W, Su SB, et al. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J Immunol 1999;163:6777-84.
- Klein C, Paul JI, Sauve K, Schmidt MM, Arcangeli L, Ransom J, et al. Identification of surrogate agonists for the human FPRL-1 receptor by autocrine selection in yeast. Nat Biotechnol 1998;16:1334-7.
- Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med 2000;191:1197-208.
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298:1911-2.
- Zhang CC, Shapiro DJ. Activation of the p38 mitogen-activated protein kinase pathway by estrogen or by 4-hydroxytamoxifen is coupled to estrogen receptor-induced apoptosis. J Biol Chem 2000;275:479-86.
- Yamagishi S, Yamada M, Ishikawa Y, Matsumoto T, Ikeuchi T, Hatanaka H. p38 mitogen-activated protein kinase regulates low potassium-induced c-Jun phosphorylation and apoptosis in cultured cerebellar granule neurons. J Biol Chem 2001;276:5129-33.
- Le Y, Oppenheim JJ, Wang JM. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev 2001;12:91-105.
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem 1997;272:6972-8.
- Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol 2006;534:1-11.
- Tsu RC, Lai HW, Allen RA, Wong YH. Differential coupling of the formyl peptide receptor to adenylate cyclase and phospholipase C by the pertussis toxin-insensitive Gz protein. Biochem J 1995;309:331-9.
- Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, et al. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem 2002;277:1332-9.
- van den Brink MR, Kapeller R, Pratt JC, Chang JH, Burakoff SJ. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J Biol Chem 1999;274:11178-85.
- Xue L, Murray JH, Tolkovsky AM. The Ras/phosphatidylinositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signaling pathway in sympathetic neurons. J Biol Chem 2000;275:8817-24.
- Kniss DA, Xie Y, Li Y, Kumar S, Linton EA, Cohen P, et al. ED(27) trophoblast-like cells isolated from first-trimester chorionic villi are genetically identical to HeLa cells yet exhibit a distinct phenotype. Placenta 2002;23:32-43.
