Distributive characteristics of Ser49Gly and Gly389Arg genetic polymorphisms of β1-adrenoceptor in Chinese Han and Dai populations1
Introduction
Human β-adrenergic receptor is a member of the 7-transmembrane superfamily of G protein-coupled receptors. β1-adrenoceptor is encoded by an intronless gene located on chromosome 10q24−26, and it was firstly cloned and sequenced in 1987[1–3]. In human heart, β1-adrenoceptor is the predominant receptor that modulates cardiac inotropy and chronotropy in response to endogenous catecholamines through coupling to Gs to activate adenylyl cyclase[4,5]. Recently, Podlowski et al identified 7 different single-nucleotide polymorphisms (SNP) in the β1-adrenoceptor gene by using polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP) analysis, and found that each SNP led to an amino acid exchange[6]. Two of these SNP have also been reported by previous studies, and are thought to be of functional significance. One of the 2 functionally important SNP is located at the amino-terminus: an A→G exchange at 145 bp (A145G) results in an amino acid substitution of Ser by Gly at residue 49 (Ser49Gly). A previous study confirmed that the Ser to Gly substitution affected agonist-promoted trafficking, and reported that the Gly49 polymorphic receptor had enhanced agonist-promoted downregulation[7]. It had also been reported that the Gly49 allele frequency was approximately 0.13–0.15 in a Caucasian American population; however, racial differences in the distribution of the Gly49 allele have not yet been reported. A previous study showed that a genetic polymorphism in the β1-adrenoceptor gene existed in patients with idiopathic dilated cardiomyopathy (IDCM) and congestive heart failure[8].
A G→C exchange at 1165 bp in the β1-adrenoceptor gene causes amino acid substitution of Gly by Arg at residue 389 (Gly389Arg). The Gly389Arg polymorphism occurs within the intracellular cytoplasmic tail near the 7-transmembrane-spanning segment of the β1-adrenoceptor. A previous in vitro study confirmed that the Gly to Arg substitution markedly altered the G-protein coupling of the β1-adrenoceptor molecule, and that the level of cAMP generated by β1-adrenoceptor activation following exposure to an agonist differed by about 3-fold for the Arg389 and Gly389 variants[9]. It seems that there are racial differences in the frequency of alleles at codon 389, the frequencies of the Arg389 allele in Caucasian Americans and African-Americans were 0.73 and 0.58, respectively[10,11]. This allele may contribute to individual and interethnic differences in drug responses, primarily through β1-adrenoceptor modulation.
China is a multicultural country; the population comprises 55 ethnic minorities in addition to the Han majority. Each of these ethnic groups has a unique genetic background. The Dai group is the 17-largest ethnic group in China, comprising more than one million people, who live chiefly in the west of Yunnan province, in South-western China[12]. Our previous studies showed that the incidence of drug metabolizing enzyme polymorphisms varies in different ethnic groups[13]. It was therefore interesting to determine whether the incidence of β1-adrenoceptor polymorphisms were concordant in 2 different ethnic populations in the same country.
β1-Adrenoceptor is an important target of many drugs and endogenous substances. A better understanding of individual and interethnic differences with respect to β1-adreno-ceptor polymorphisms may help us to explain intraindividual differences in drug response and disease susceptibility. Therefore, in the present study we investigated the frequencies of the main mutants of the β1-adrenoceptor gene in Han and Dai Chinese populations. Because we consider that polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis is currently one of the most reliable methods for population genotyping, PCR-RFLP methods for Ser49Gly and Gly389Arg genotyping of the β1-adrenoceptor gene were used.
Materials and methods
Subjects The study protocol was approved by the Ethics Committee of Xiang-Ya School of Medicine, Central South University. A total of 225 Han Chinese (123 men, and 102 women) living in Hu-nan province and 175 Dai Chinese living in Yunnan province (89 men, and 86 women) were recruited for the present study. The subjects were unrelated healthy volunteers aged 18–21 years, and all participated after giving their written informed consent. All of the Han subjects were students from Xiang-Ya School of Medicine, Central South University, and the Dai subjects were students from the Dali Nationality School, Yunnan province. None of the subjects had any abnormalities according to their medical records, routine laboratory tests, physical examinations, or electrocardiography. Samples (5 mL per subject) of peripheral vein blood were collected for genotyping analysis (Table 1).
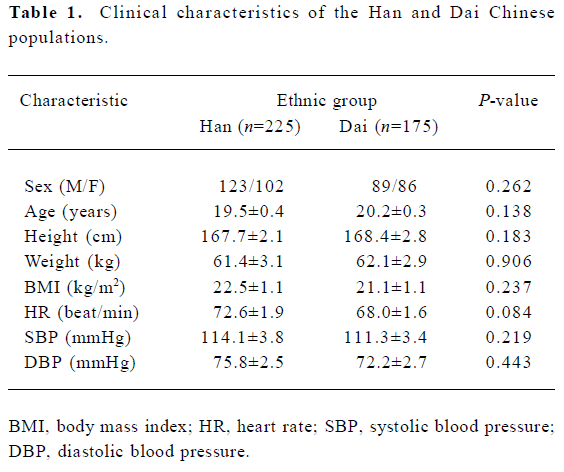
Full table
Genotyping procedures for β1-adrenergic receptor Genomic DNA was extracted from peripheral lymphocytes with phenol-chloroform followed by ethanol precipitation[14]. Genotyping analysis was carried out by using the PCR-RFLP assay. The PCR procedure for the β1-adrenergic receptor gene was performed as described previously with minor modifications[15]. For the Ser49Gly locus, we used the following primer pairs: the sense primer P1, 5'-CCGGGCTTCTGG-GGTGTTCC-3', and the antisense primer P2, 5'-GGCGAGGTG-ATGGCGAGGTAGC-3'. The Gly389Arg polymorphic locus was amplified by using the sense primer P3, 5'-CATCATGGG-CGTCTTCACGC-3', and the antisense primer P4, 5'-TGGGC-TTCGAGTTCACCTGC-3'. The amplified DNA fragments including the Ser49Gly or Gly389Arg polymorphic sites were separately digested with Eco0109I (TaKaRa Biotech, China) or BcgI (England Biolabs, Beverly, USA) at 37 °C for 8 h. The different patterns produced by the digested fragments were visualized on 2% agarose gels stained with ethidium bromide. Following the amplifications, some of the PCR product was purified and sequenced to confirm the accuracy of the RFLP assay.
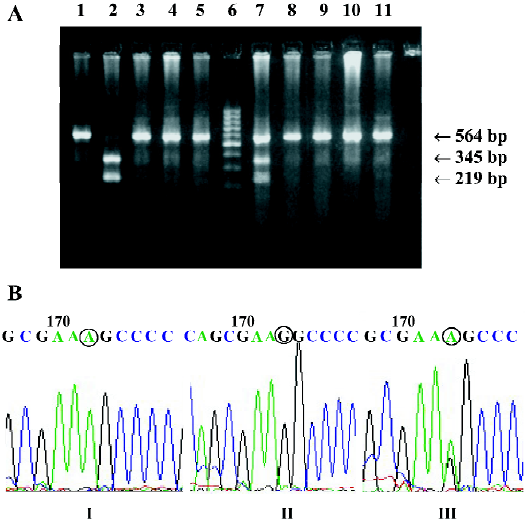
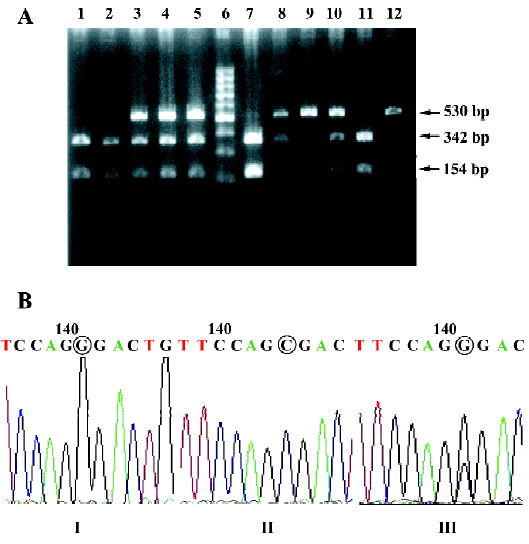
Ser49Gly and Gly389Arg point mutations The Ser49Gly point mutation gave rise to an Eco0109I cleavage site. The PCR product of the Gly49 allele contained a unique site for restriction by Eco0109I. The PCR product of the Gly49 allele contained a unique site for restriction by Eco0109I. The Ser49Gly mutant allele had 3 bands, 564 bp, 345 bp, and 219 bp, which became apparent when digested fragments were detected with an ultraviolet detector (Figure 1). The Gly389Arg substitution gave rise to a BcgI cleavage site. The Gly389Arg mutant had 3 bands, 530 bp, 342 bp, and 154 bp. BcgI cleaves twice, excising its recognition site, which accounts for the 34 bp discrepancy in the fragments generated (Figure 2). When the PCR-RFLP genotyping assay was repeated for randomly selected samples, the result of the second genotyping of each sample was identical to the first in every case, thus verifying the reproducibility of the genotyping results.
Statistical analysis Data analysis was performed using SPSS (version 10.0 for Windows, SPSS, Chicago, IL, USA). A two-tailed value of P<0.05 was considered statistically significant. The frequencies of each allele polymorphism were calculated based on the observed number of different alleles (49Ser or 49Gly, or 389Gly or 389Arg) in each ethnic group. Hardy-Weinberg equilibrium and possible differences in allele frequencies between the 2 populations were tested by using the χ2-test.
Results
In the present study, we found that the 2 single nucleotide polymorphisms causing the Ser49Gly and Gly389Arg mutants of the β1-adrenoceptor existed in both the healthy Han and Dai Chinese populations.
The allele and genotype frequencies for the β1-adrenoceptor Ser49Gly and Gly389Arg polymorphisms in Han and Dai populations are summarized in Table 2. The allelic frequencies for Gly49 and Arg389 were 16.2% and 76.4% in the Han population and 14.6% and 75.7% in the Dai population, respectively. The allele and genotype frequencies for the 2 different populations were in Hardy-Weinberg equilibrium as tested by comparisons of the frequencies found versus the frequencies expected on the basis of the proportion represented by the more frequently occurring allele. There was no difference between the Han and Dai populations with respect to either of the allele frequencies. Compared with data in the literature regarding the 2 polymorphisms in healthy subjects from different ethnic groups, significant interethnic differences in the incidence of the Arg389 polymorphism were found between a Chinese and an African American population; however, the frequencies of the Ser49Gly polymorphism were not significantly different in these 2 populations[11].
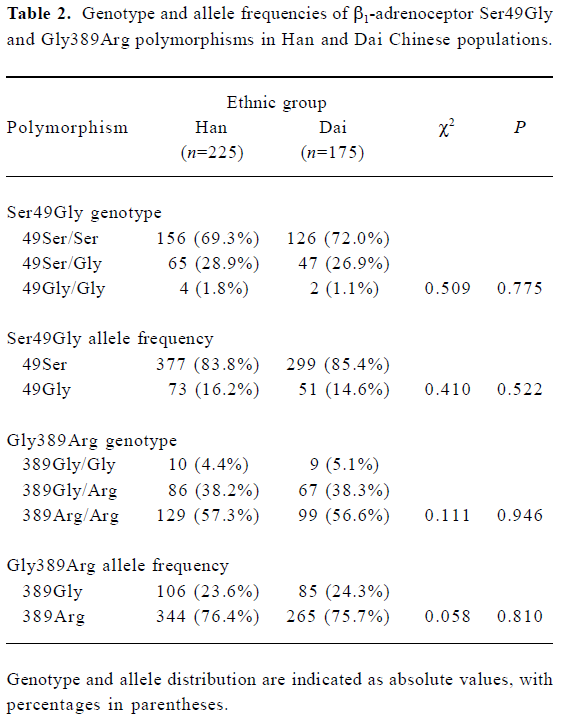
Full table
Discussion
Τhe β1-adrenoceptor is an important target for many drugs and endogenous substances[16]. In the present study, we carried out the first investigation of the frequencies of the Ser49Gly and Gly389Arg genetic polymorphisms of β1-adrenoceptor in Han and Dai Chinese populations and found the frequencies for both polymorphisms were not significantly different in the 2 populations; however, the frequency of the Gly389 variant appeared to be significantly lower in the Han and Dai Chinese populations than in an African-American population.
In the present study, we found that the allelic frequencies of Gly49 and Arg389 were approximately 14.6%–16.2% and 75.7%–76.4% in the combined Han and Dai populations, respectively. Gly389 is considered to be the wild type, and was first cloned by Frielle et al as a Gly389-containing receptor[1].
The Dai ethnic group is one of many minority groups in China who have unique characteristics with respect to genetic make-up, culture, diet and environment[12]. Previous studies carried out by our laboratory have found that interethnic differences with respect to polymorphisms in drug metabolizing enzymes such as CYP2C19 exist between Han and Dai populations. He et al reported that the frequency of the CYP2C19*3 allele in a Dai population was significantly lower than that in a Han population[13]. In the present study, we found that there was no significant difference in the frequencies of the β1-adrenoceptor Ser49Gly and Gly389Arg polymorphisms between the two ethnic groups. An explanation for the difference between the 2 enzymes with respect to the level of polymorphism in different populations is that β1-adrenoceptor may well be more conserved throughout different populations than drug metabolizing enzymes. Many drugs that have effects mediated by β1-adrenoceptor have obvious interethnic differences in their effects[17]. If such a difference was also found between Han and Dai populations, the differences would be unlikely to be due to β1-adreno-ceptor polymorphisms, but would rather be due to genetic variations in drug-metabolizing enzymes and the effects of environmental factors.
A previous study reported that the incidence of the Ser49Gly amino acid substitution was 16.2% in patients with IDCM, but zero in a healthy population[6]. In contrast, we found that the frequency of the Gly49 allele was approximately 14.6%–16.2% in healthy subjects, which is consistent with the results of other studies, in which the frequency of the Gly49 allele was found to be 2%–15% in healthy subjects[8,15,18]. Because one study in which the Gly49 allele was only detected in patients with IDCM used only a small sample size (37 patients), the resulting in low statistical power might have produced a false result. However, several other studies that used a large sample size have also found that there was a higher frequency of the Gly49 allele in patients with ICDM relative to healthy subjects[6,8,15].
An in vitro mutagenesis study of the codon 389 polymorphism revealed that the Arg389 receptor form had nearly two-fold greater basal and 3-fold greater agonist-mediated adenylyl cyclase activity[9], which suggests that the Gly389 mutation produces a hypoactive β1-adrenoceptor, and that a higher frequency of the Gly389 allele could contribute to decreased sensitivity to β-blockers in African-Americans. However, we found no ethnic differences in the frequency of the β1-adrenoceptor Gly389Arg polymorphism between Han and Dai Chinese populations in the present study, indicating that this polymorphism is unlikely to account for any ethnic differences in sensitivity to β-blockers between these two populations.
In conclusion, we found that the β1-adrenoceptor Ser49Gly and Gly389Arg mutations both existed in both Han and Dai Chinese populations, and that the frequencies of the two polymorphisms were not significantly different in these two ethnic groups.
References
- Frielle T, Collins S, Daniel KW, Caron M, Lefkowltc RJ. Cloning of the cDNA for the human β1-adrenergic receptor. Proc Natl Acad Sci USA 1987;84:7920-4.
- Yang-Feng TL, Xue F, Zhong W, Cotecchia S, Frielle T, Francke U, et al. Chromosomal organization of adrenergic receptor genes. Proc Nat Acad Sci 1990;87:1516-20.
- Collins S, Ostrowski J, Lefkowitz RJ. Cloning and sequencing analysis of the human β1-adrenergic receptor 5-flanking promoter region. Biochim Biophys Acta 1993;1172:171-4.
- Strader CD, Fong TM, Tota MR, Underwood D. Structure and function of G protein-coupled receptors. Annu Rev Biochem 1994;63:101-32.
- Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med 1998;158:S146-53.
- Podlowski S, Wenzel K, Lutter HP, Muller J, Bramlage P, Baumann G, et al. β1-adrenoceptor gene variations: a role in idiopathic dilated cardiomyopathy? J Mol Med 2000;78:87-93.
- Rathz DA, Brown KM, Kramer LA, Liggett SB. Amino acid 49 polymorphisms of the human beta1-adrenergic receptor affect agonist-promoted trafficking. J Cardiovasc Pharmacol 2002;39:155-60.
- Tesson F, Charron P, Peuchmaurd M, Nicaud V, Cambien F, Tiret L, et al. Characterization of a unique genetic variant in the β1-adrenoceptor gene and evaluation of its role in idiopathic dilated cardiomyopathy. CARDIGENE Group. J Mol Cell Cardiol 1999;31:1025-32.
- Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human β1-adrenergic receptor. J Biol Chem 1999;274:12670-4.
- Moore JD, Mason DA, Green SA, Hsu J, Liggett SB. Racial difference in the frequencies of cardiac β1-adrenergic receptor polymorphisms: analysis of c145A→G and c1165G→C. Hum Mut 1999;14:271.
- Xie HG, Dishy V, Sofowara G, Kim RB, Landau R, Smiley RM, et al. Arg389Gly β1-adrenoceptor polymorphism varies in frequency among different ethnic groups but does not alter response in vivo. Pharmacogenetics 2001;11:191-7.
- Etler DA. Recent developments in the study of human biology in China: a review. Hum Biol 1992;25:64567-85.
- He N, Yan FX, Huang SL, Wang W, Xiao ZS, Liu ZQ, et al. CYP2C19 genotype and S-mephenytoin 4'-hydroxylation phenotype in Chinese Dai populations. Eur J Pharmacol 2002;58:15-8.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989.
- Maqbool A, Hall AS, Ball SG, Balmforth AJ. Common polymorphisms of β1-adrenoceptor: identification and rapid screening assay. Lancet 1999;353:897.
- Hein L, Kobilka BA. Adrenergic receptors: from molecular structure to in vivo function. Trends Cardiovasc Med 1997;7:137-45.
- Humma LM, Puckett BJ, Richardson HE, Terra SG, Andrisin TE, Lejeune BL, et al. Effects of β1-adrenoceptor genetic polymorphisms on resting hemodynamics in patients undergoing diagnostic testing for ischemia. Am J Cardiol 2001;88:1034-7.
- Borjesson M, Magnusson Y, Hjalmarson A, Andersson B. A novel polymorphism in the gene coding for the β1-adrenergic receptor associated with survival in patients with heart failure. Eur Heart J 2000;21:1853-8.
