Inhibition of tumor growth and metastasis with antisense oligonucleotides (Cantide) targeting hTERT in an in situ human hepatocellular carcinoma model1
Introduction
Telomerase is a unique ribonucleoprotein enzyme responsible for adding telomeric repeats onto the 3' ends of chromosomes[1,2]. Telomerase plays an important role in the development of cellular immortality and oncogenesis. Previous published studies have shown that telomerase activity is found in 85%–90% of all human tumors, but not in their adjacent normal cells. This makes telomerase a good target not only for cancer diagnosis, but also for the development of novel therapeutic agents[3,4].
A series of experimental strategies have been employed to block either telomerase function or expression, both in vitro and in vivo in several tumor cell lines derived from a number of species[5,6]. Antisense oligonucleotide (ASODN) is a new class of antineoplastic agents that can prevent the initiation and progression of specific human cancers when targeted to appropriate molecular targets[7,8]. Since the first antisense drug Vitravene was approved by the American Food and Drug Administration (FDA) in 1998, it has been our hope that this class of compound will represent a whole new class drug with potential therapeutic application against a variety of disease, including cancer. Human telomerase reverse transcriptase (hTERT) is a catalytic subunit of telomerase. There are many published studies which have found that more than 90% of examined tumors show telomerase activity in connection with the expression of the activity-limiting component hTERT[9,10]. The inhibition of telomerase activity in tumor cells can lead to telomere destabilization and consequently to growth inhibition and cell death. Therefore, targeting the catalytic subunit of hTERT represents a promising approach for diminishing telomerase function. Phosphoro-thioate antisense oligonucleotides targeted to hTERT had been studied and has been found to have growth inhibitory efficacy to cancer cells in vitro[11,12], but only few detected ASODN activity in animal models[13,14]. Our previous study had shown that an ASODN complementary to hTERT, Cantide, had a specific inhibitory effect on tumor cell growth after in vitro treatment, down-regulated hTERT mRNA expression and telomerase activity, and triggered apoptosis through the activation of the caspase family[15].
In the present study, we used an in situ human hepatocellular carcinoma (HCC) model to further assess the effect of Cantide in in vivo growth of HCC in mice and the combined effect of Cantide with 5-fluorouracil (5-FU). Our findings demonstrated that systemic administration of phosphorothioate antisense ODN to hTERT mRNA resulted in a significant growth inhibition of in situ HCC tumors and prevented tumor recurrence in the liver and metastasis in the lung.
Materials and methods
Oligonucleotides and drug Antisense phosphorothioate oligonucleotide Cantide (5'-ACTCACTCAGGCCTCAGACT-3') and sense phosphorothioate oligonucleotide (5'-AGTCT-GAGGCCTGAGTGAGT-3') were synthesized by an Applied Biosystems Model 391 DNA synthesizer on solid supports using Oligo Pilot II DNA (Amersham-Pharmacia, Piscataway, NJ, USA) and purified by high-performance liquid chromatography (HPLC) (Waters Delta Prep 4000, Milfordd, MA, USA) with SOURCE 15Q (Amersham Pharmacia, Piscataway, NJ, USA), the purity of oligonucleotides was over 95%. The sense sequence was used as a control. 5-Fluorouracil was purchased from the Shanghai Donghaipu Pharmaceuticals Company (Shanghai, China) as a positive control. An radiation immunoassay (RIA) kit was purchased from the China Institute of Atomic Energy (Beijing, China).
Animals and in situ human hepatocellular carcinoma model Experiments were carried out in virgin female Balb/c mice raised at the PLA 202 hospital. All animal studies were conducted in accordance with the highest standards of animal care as outlined in the NIH guide for the Care and Use of Laboratory Animals. Human hepatocellular carcinoma (HCC) high metastasis tumor line HCM-Y89, established in 1989 by PLA 202 Hospital, was derived from a HCC surgical specimen, and has been maintained by serial transplantation in animals. Briefly, the HCM-Y89 tumor was cut into 1 mm×1 mm×1 mm tissues, BALB/c mice (4–6 weeks old, weighing 18–22 g) were anesthetized with propofol (2 mg/kg, intravenously). A small incision was made in the left liver and a fragment of tumor was implanted. The incision was closed using a Ethicon black braided silk suture. Twenty days later, the mice were killed by cervical dislocation and the tumors were removed. A histological evaluation of tumor tissues was performed, organs were fixed in neutral buffered 10% formalin, processed by standard methods, embedded in paraffin, sectioned and stained with H&E. This model showed various features in clinical liver cancer patients including local growth, regional invasion, spontaneous intrahepatic, lymph nodes and pulmonary metastasis, peritoneal seeding with bloody ascites, and secretion of alpha-fetoprotein in recipient animals.
Treatment of in situ HCC xenograft with cantide and anticancer drug Two days after the in situ HCC models were established, mice were injected by iv with saline (vehicle control), Cantide with 12.5, 25, 50, 75 mg·kg-1·d-1 or 5-FU10 mg·kg-1·d-1 for 20 d, either alone or in combination. The body weight and general physical status of the animal were recorded daily. At the endpoint of the study, mice were killed by cervical dislocation and the tumors were removed and weighed. Tumor sizes were monitored with calipers, the tumor volume (V, mm3) was calculated as (L×W2)/2, where L=length (mm) and W=width (mm). The percentage of tumor growth inhibition was calculated as:
Inhibitory rate (%)=(Wcontrol–Wtreat)/Wcontrol×100. Tissues for histopathological analysis were fixed in 10% buffered formalin.
Determination of tumor recurrence and metastasis The in situ HCC model was established according to above method, when the xenograft tumor had grown to 5 mm×5 mm×5 mm. An operation was then performed to remove the tumor. Three days later, saline and Cantide at 12.5, 25, 50, 75 mg·kg-1·d-1, or 5-FU 10 mg·kg-1·d-1 were administered by iv for 20 d. Tumor sizes were recorded as mentioned earlier. A histological evaluation of microscopic recurrence and metastasis was performed for the liver and lung, and tissues were fixed in 10% buffered formalin.
Histophatological analysis Tissues were excised and fixed in 10% buffered formalin. Representative fragments were embedded in paraffin, 5-μm sections were obtained and stained with H&E for microscopic observations.
Detection of plasma AFP concentration Animal serum was collected after the mice were killed. The plasma AFP concentrations were detected by RIA according to the manufacturer’s instructions.
Statistical analysis Results of the quantitative studies were expressed as mean±SD. Student’s t-test was used to determine the significance of differences between the 2 treatment groups. Multiple comparisons were made using one-way analysis of variance (ANOVA), and post-tests comparing different treatment means were conducted using Fisher’s test. Differences were considered significant for P<0.01
Results
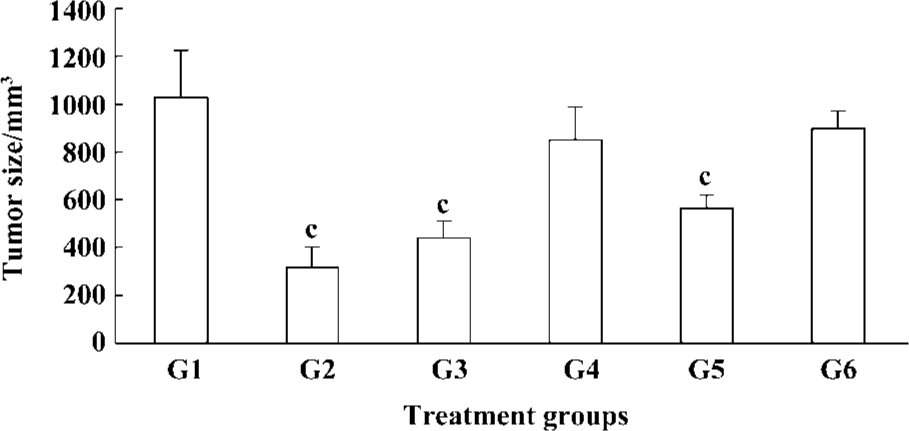
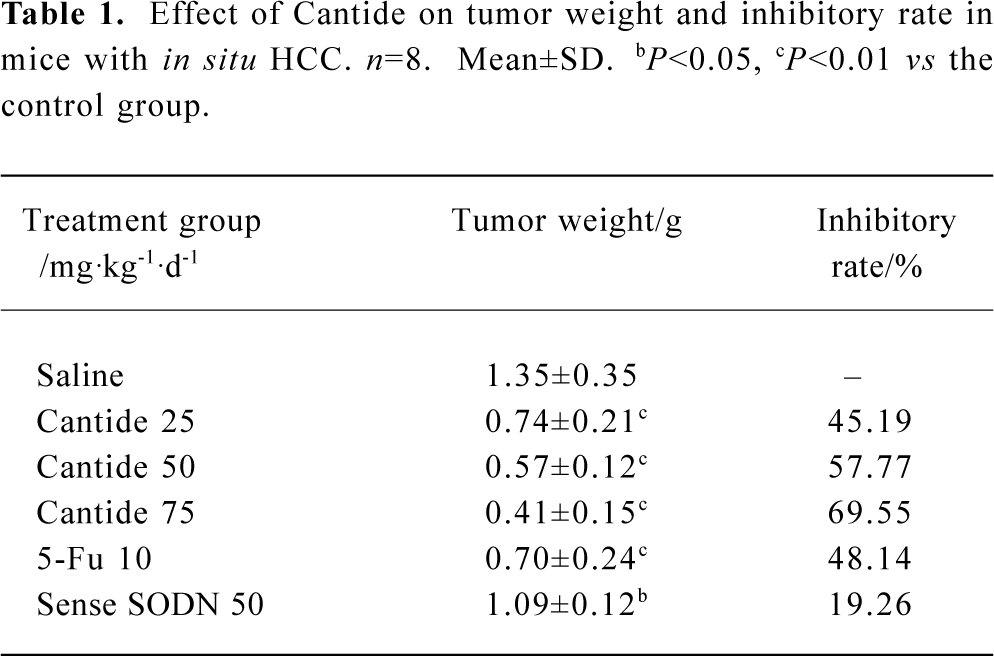
In vivo effects of Cantide treatment on in situ hepatocellular carcinoma xenograft growth The proposed role of the telomerase hTERT subunit has led us to investigate the pharmacological activity of phosphorothioate oligonucleotides targets to hTERT in animal models on tumor growth. In the present study, we used an in situ human hepatocellular carcinoma model in mice to evaluate the activity of Cantide. In order to exclude the nonspecific effect of oligonucleotides, we used sense sequence as a control. After establishing the model 2 d later, Cantide with different doses, sense oligonucleotide with 50 mg·kg-1·d-1, 5-Fu with 10 mg·kg-1·d-1 and saline were administered by intravenous (iv) for 20 d. Tumors were removed after the mice were killed. The tumors were then measured and weighed. The results showed that 75 and 50 mg·kg-1·d-1 Cantide treatment resulted in a significant inhibition of tumor growth compared to sense or saline-treated mice. The highest inhibitory efficacy was a 69.55% contrast to the saline group, but for the sense oligonucleotide, the inhibitory rate was only a 19.26% contrast to the saline group. The inhibitory rate of 50 mg·kg-1·d-1 was 57.77% compared to the sense treatment group (P<0.01). The effect of Cantide was dose-dependent (Table 1). The mean body weight of the mice were not significantly different from the beginning of the study and between the groups (data not shown). Figure 1 shows the final tumor volumes after a 20-d treatment, which showed a significant decrease in the Cantide treatment group compared to the saline control group (P<0.01). However for the sense oligonucleotide treatment group, there was no significant decrease in tumor volume compared to the saline group (P>0.05). No inflammatory infiltrate was noted surrounding the solid tumor (data not shown).
Full table
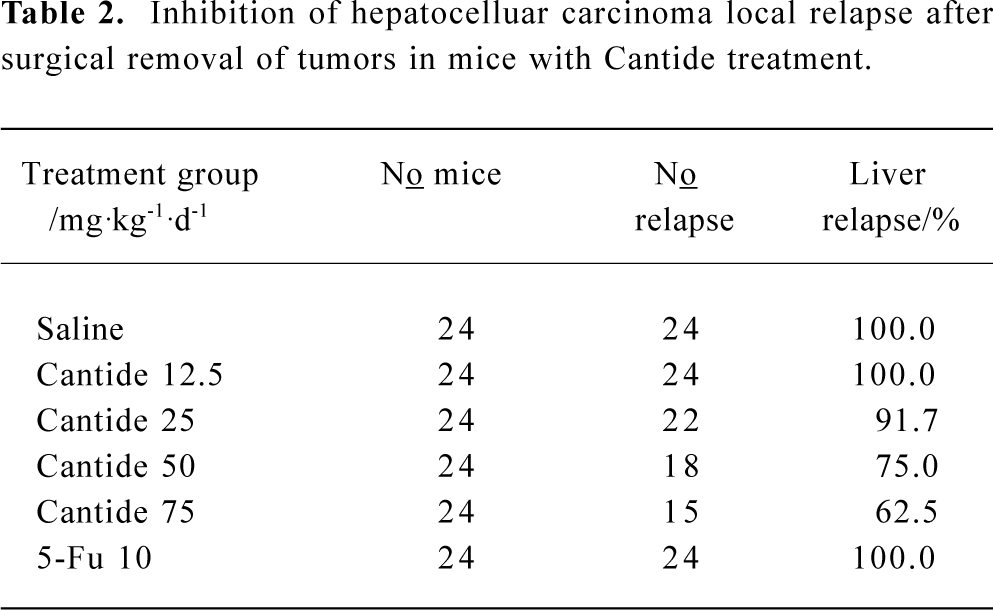
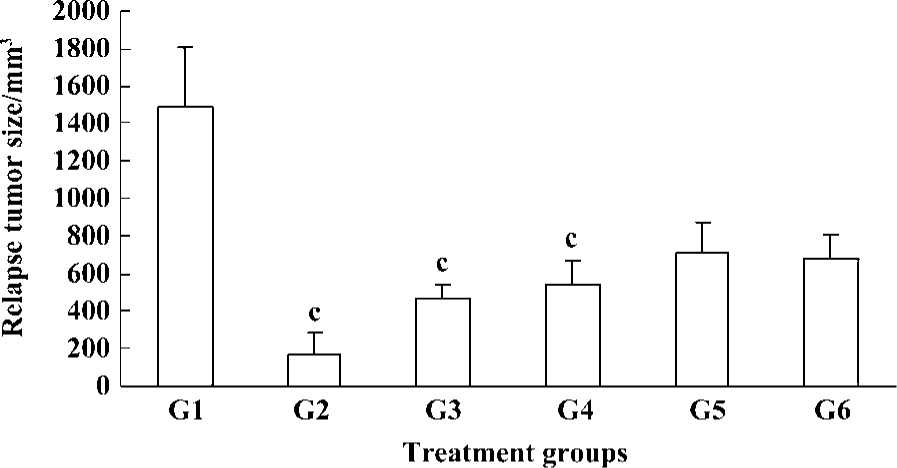
To determine the effect of Cantide on preventing tumor recurrence and metastasize after surgical removal of the tumors, a highly metastatic HCC model was established in the mice liver according to the above method. When the xenograft tumor had grown to 5 mm×5 mm×5 mm, the mice underwent an operation to remove the tumors; 3 d later, animals were injected with different doses of Cantide or saline by iv for 20 d and then checked for a relapse in the liver. Table 2 shows how the Cantide 75 and 50 mg·kg-1·d-1 treatment group could significantly inhibit tumor recurrence in livers after operation. However, there was no statistically significant difference in tumor recurrence in livers treated with 25 mg·kg-1·d-1 and 12.5 mg·kg-1·d-1 in the Cantide treatment group compared to the saline treatment group. 5-FU could not prevent tumor relapse after operation. The relapse tumor volumes also decreased significantly in the Cantide treatment group compared to the Saline control group (Figure 2). No signs of treatment-related toxicity such as inflammation, bleeding, hepatomegaly and splenomegaly or weight loss was observed in the animals over the course of the study.
Full table
Inhibitory effects of Cantide on tumor metastasis in lung Tumor metastasis in the lung was also monitored after surgical removal of the tumors from the mice livers and treatment with Cantide or saline for 20 d. Cantide can also prevent tumor metastasis in the lung after operating on the liver. The results found that only 10 and 14 of the 24 mice tested showed evidence of tumor metastasis in the lung with 75 and 50 mg·kg-1·d-1 in the Cantide treatment groups, respectively (Table 3). A histological analysis of the lungs showed that the metastatic areas markedly decreased in mice treated with 75 and 50 mg·kg-1·d-1 Cantide compared to the other group, where 5-FU had no effect on tumor metastasis (data not shown).
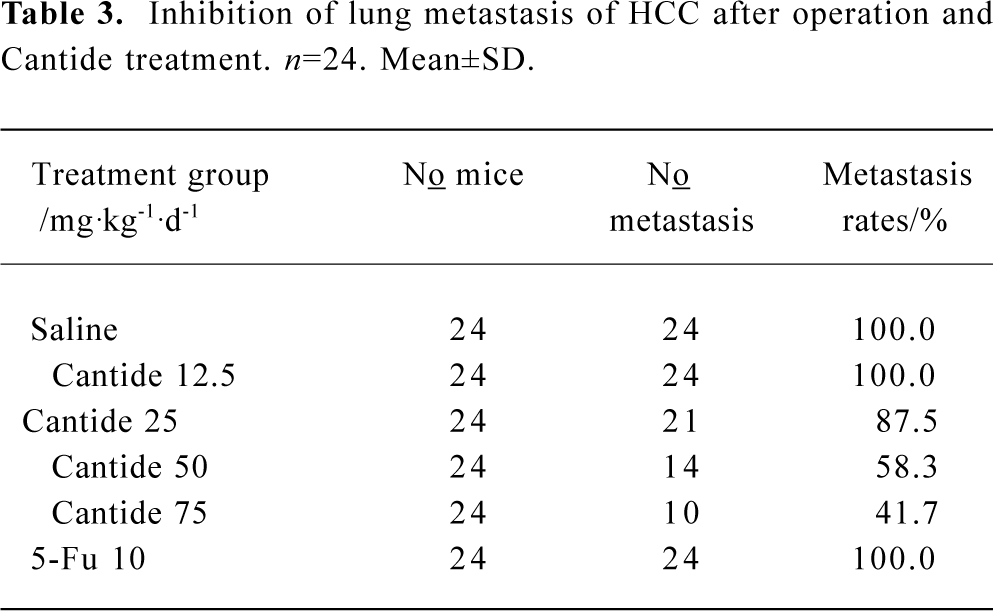
Full table
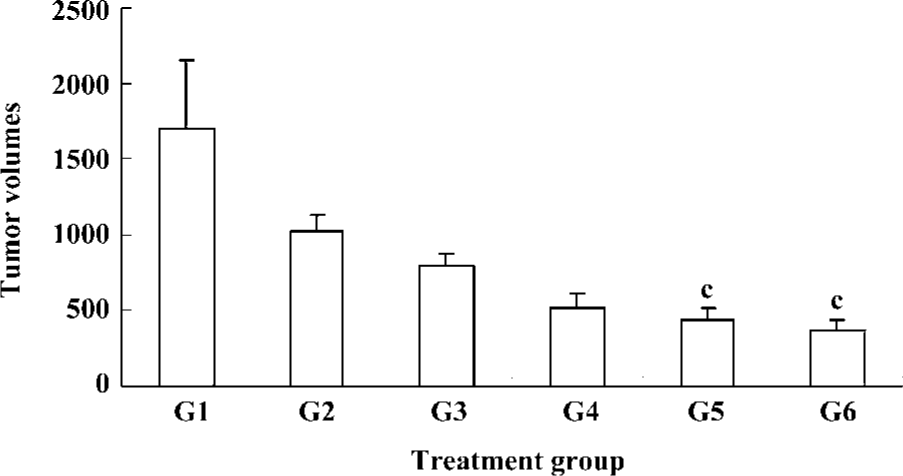
Effects of Cantide combined with 5-FU on xenograft tumor growth Several studies have shown that antisense oligonucleotides combined with chemotherapeutics can increase drug sensitivity[16,17]. In order to evaluate whether a combination effect would occur when Cantide was combined with a chemotherapy drug, Cantide was co-administered with 5-FU by iv for 20 d to mice bearing with in situ HCC tumors. The mice were killed and tumor volumes and weight were measured after the 20-d treatment. Table 4 shows that after the 20-d treatment, the tumor weight decreased in the combination treatment group compared to either agent alone treatment group. The tumor growth inhibitory rate also showed significant difference compared to either agent alone treated group (Table 4). Figure 3 shows that at the endpoint of treatment in the combination treatment group, a significant decrease in tumor volumes with respect to tumors growth in either Cantide or the 5-FU alone treatment group was induced.
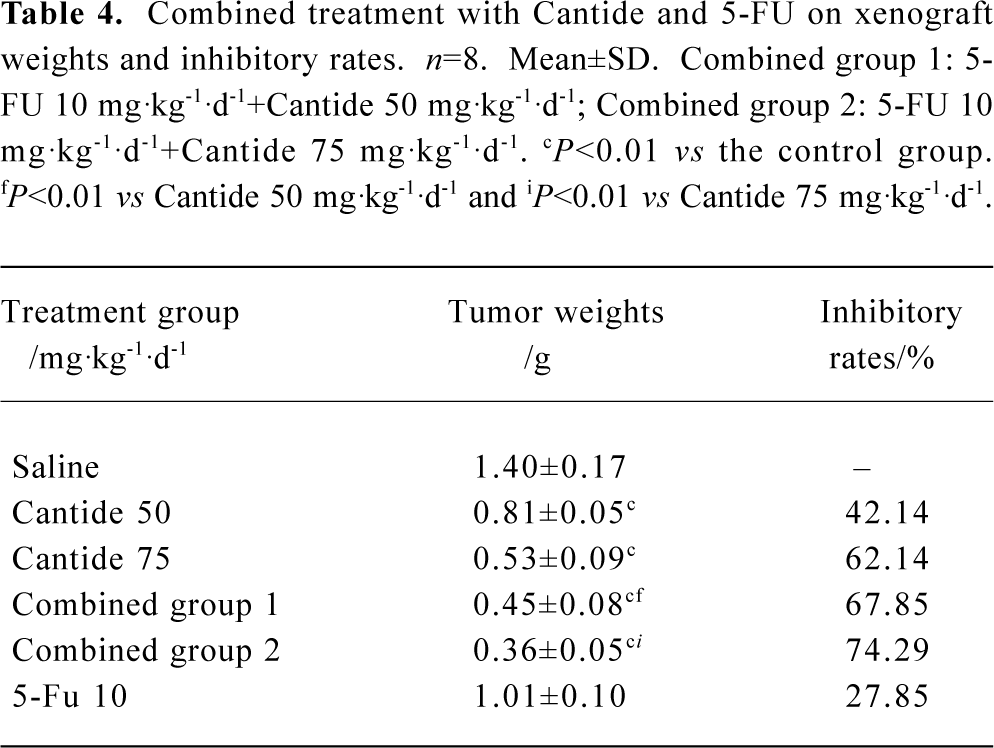
Full table
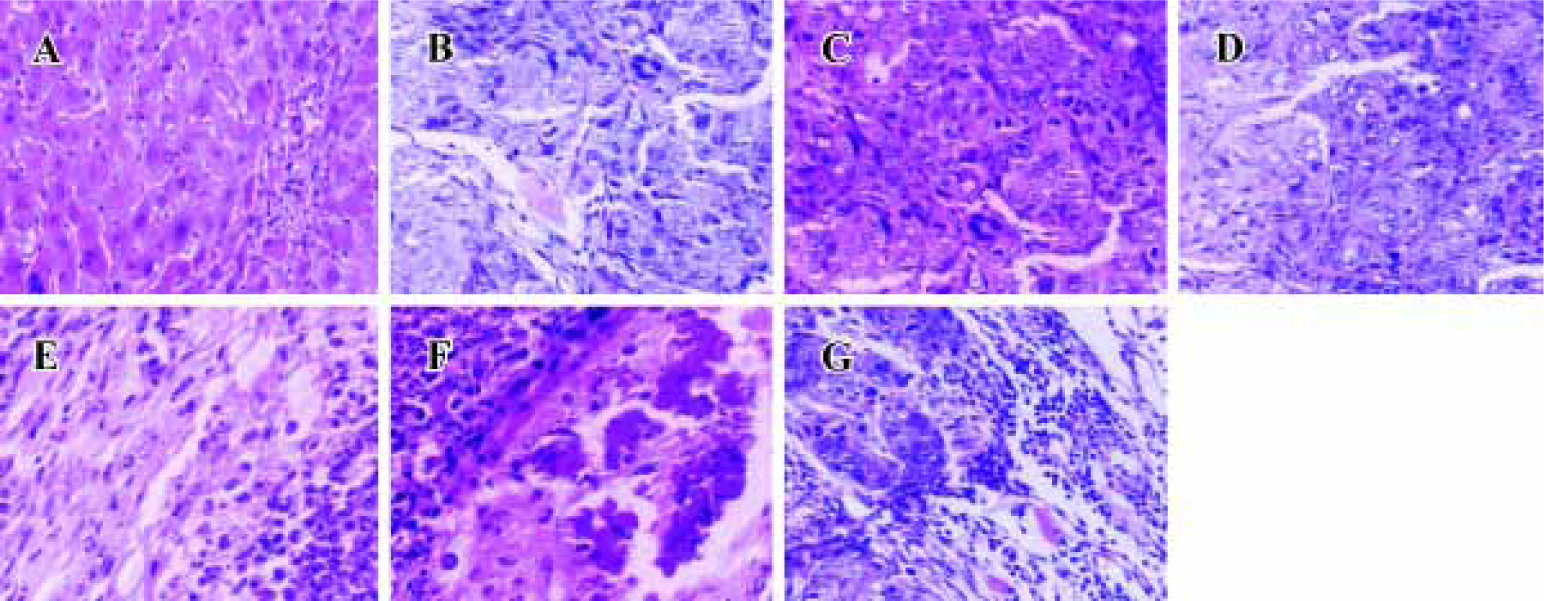
Histopathological analysis Morphology of tumors from antisense ODN, sense ODN or saline-treated mice was evaluated by H&E staining of histological sections. Tumors were excised at the endpoint of treatment of each protocol. Figure 4 shows representative sections of tumors from each experimental protocol. Tumors from mice treated with Cantide or combined with 5-FU, showed a marked increase in the necrotic area and tumor cells fibrosis compared to saline-treated or normal animals. A large percentage of tumor mass from combination-treated mice was necrotic and fibrotic compared to either agent alone treated mice.
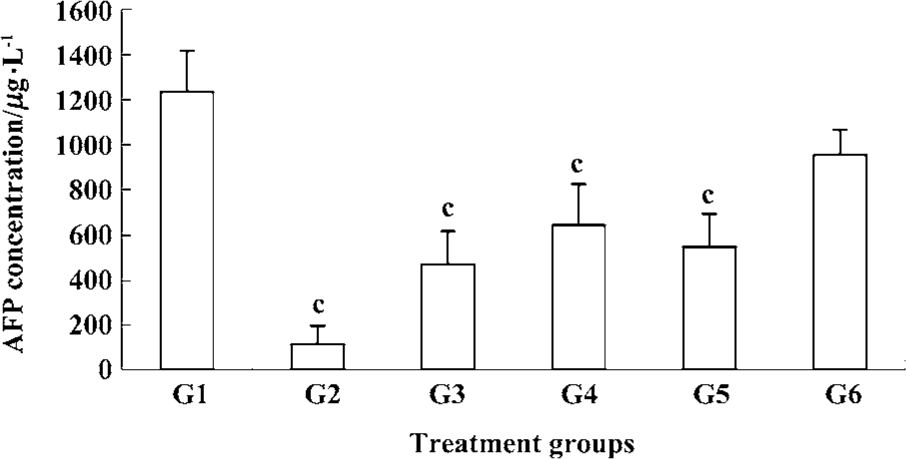
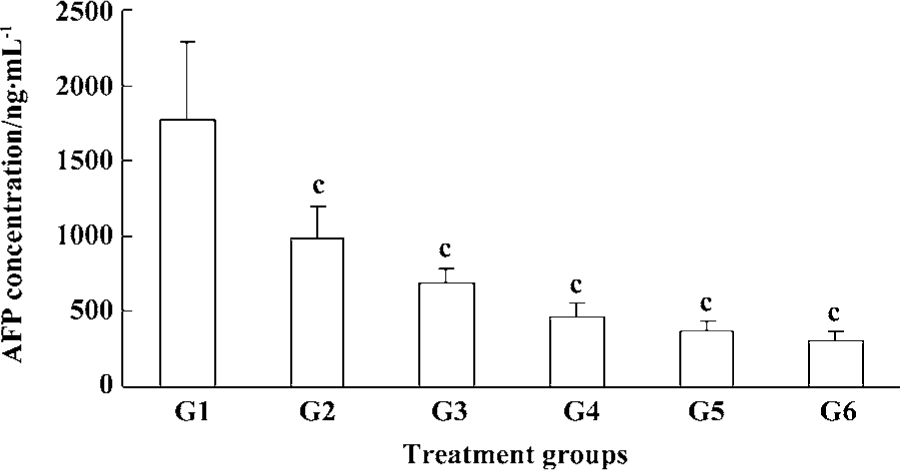
Inhibition of plasma AFP secretion with Cantide treatment Alpha-fetoprotein (AFP) is usually expressed at high concentrations in fetal liver, gastrointestinal tract and the yolk sack. It is transcriptionally down-regulated after birth, frequently re-expressed in HCC,and is a good tumor marker of HCC[13]. We used RIA to detect serum AFP concentration at the endpoint of treatment. Figure 5 shows how Cantide can significantly decrease AFP secretion at 75, 50, and 25 mg·kg-1·d-1 compared to the saline group. AFP concentration was also correlated with tumor size (data not shown). The plasma AFP was also detected after treatment with Cantide combined with 5-FU. Figure 6 shows how Cantide combined with 5-FU could increase AFP secretion in plasma. In the two combination treatment groups, plasma AFP concentration decreased compared to either the Cantide or 5-FU alone treatment groups, possibly because there were fewer liver tumor cells in the combined treated mice and reduced circulating AFP.
Discussion
In the present study we have demonstrated that systemic administration of phosphorothioate antisense ODNs to hTERT mRNA results in a significant growth inhibition of the in situ HCC model in mice. It is worth noting that our experimental model of HCC has some advantages compared to the tumor cell inoculation model. First, the tumor line originated from human HCC; it completely kept the human HCC tissue structure and retained its AFP secretion ability. It also retained the original sensitivity to the antitumor drug, simulated the clinical features of patients with HCC such as the liver, lung and lymph nodes metastasis, and bloody ascites. Second, the pathological evidence suggests that this model exhibits various features seen in clinical HCC patients, so the reaction to antitumor drug can reflect the true clinic results by this model and more accurately to reflect the clinical results of patients. Therefore, our model system has provided a unique tool for exploring in vivo antitumor activity of ODN.
We have demonstrated that the antitumor effect of Cantide was because of a specific antisense effect. The inhibition of tumor growth by Cantide was dose-dependent and no abrogation of tumor proliferation was observed in any of the control groups. Another important finding of our present study is that Cantide can effectively prevent tumor recurrence and metastasis. Tumor recurrence and metastasis is a problem encountered during tumor treatment. At present, very few chemotherapeutics can prevent tumor relapse and metastasis after an operation. Pastorino’s[18] study indicates that an antisense targeting to c-myc can inhibit tumor growth and metastasis. In the present study we demonstrated that Cantide effectively suppressed tumor metastasis in lungs with 75 mg·kg-1·d-1 treatment. Lung metastasis was found in only 10 mice of the 24 mice (41.7%). Cantide can also prevent local recurrence after an operation. An important factor contributing to these results is that Cantide might inhibit telomerase activity[15], thus the residue of tumor cells after an operation has less ability to proliferate.
AFP is a secretary protein that is heterogeneously glycosylated. AFP is usually expressed at high concentrations in fetal liver, gastrointestinal tract, and the yolk sack. It is transcriptionally down-regulated after birth and frequently re-expressed in HCC, therefore used as a diagnostic marker for the tumor. Serum AFP is useful not only for diagnosis, but also as a prognostic indicator for HCC patients. AFP mRNA has been proposed as a predictive marker of HCC cells disseminated into the serum circulation and for metastatic recurrence[19,20]. We detected serum AFP concentration after the surgical removal of tumors and their treatment with Cantide. The results showed that serum AFP secretion was significantly inhibited in a dose-dependent manner in the Cantide treatment groups compared to the saline group. The AFP concentration was also lower than 5-FU group. The serum AFP concentration and the relapse of tumor volume had a good dependent relationship, which was correlated with AFP as a marker for HCC recurrence and metastasis.
In the past decade, several ASODN have been developed and tested in preclinical and clinical studies. Many have found convincing in vitro reduction in target gene expression and promise activity against a wide variety of tumors[21,22]. However, because of the multigenic alterations of tumors, the use of ASODN as a single agent does not seem to be effective in the treatment of malignancies. Antisense therapy that interferes with signaling pathways involved in cell proliferation and apoptosis are particularly promising in combination with conventional anticancer treatment; several preclinical studies have also been performed using antisense oligonucleotides in combination therapy on human tumors[23,24]. Our previous study showed that Cantide inhibited tumor cells growth through triggered tumor cells apoptosis[15], and increasing 5-FU sensitivity in HepG2 cells in vitro (data not shown). Zhang and He also show an increased sensitivity to cisplatin caused by hTERT ASODN treatment in leukemia cells[25]. However, to our knowledge, no published studies have been performed to detect the in vivo activity of hTERT ASODN in combination with chemotherapy drugs. We investigated the combinational activity of Cantide with 5-FU in the in situ HCC model. The results indicate that 75 or 50 mg·kg-1·d-1 Cantide combined with 10 mg·kg-1·d-1 5-FU significantly suppresses in situ HCC growth in mice. At the endpoint of the treatment, the tumor weight was much lighter than either agent alone treatment (Table 4). These results suggest that the combination of Cantide with 5-FU might sensitize patients with late-stage HCC to chemotherapy.
In summary, we have for the first time directly addressed the potential therapeutic role of Cantide in an in situ human HCC model. Significant inhibition of HCC growth was achieved by combining Cantide with 5-FU, indicating that combined targeted therapy with conventional cytotoxic agents are required for the prevention of tumor growth.
References
- Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol 1999;180:10-8.
- Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, et al. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol 2002;8:357-62.
- Granger MP, Wright W, Shay JW. Telomerase in cancer and aging. Crit Rev Oncol Hematol 2002;41:29-40.
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer 1997;33:787-91.
- Norton JC, Piatyszek MA, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase activity by peptide nucleic acids. Nat Biol 1996;14:615-9.
- Zhang RG, Wang XW, Yuan JH, Xie H. Human hepatoma cell telomerase activity inhibition and cell cycle modulation by its RNA component antisense oligodeoxyribonucleotides. Acta Pharmacol Sin 2000;21:742-6.
- Bennett CF, Cowsert LM. Application of antisense oligonucleotides for gene functionalization and target validation. Curr Opin Mol Ther 1999;1:359-71.
- Banerjee D. Technology evaluation: G-3139. Curr Opin Mol Ther 1999;1:404-8.
- Counter CM, Meyerson M, Eaton EN, Euisen LW, Caddle SD, Haber DA, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene 1998;16:1217-22.
- Yang Y, Chen Y, Zhang C, Huang H, Weissman SM. Nucleolar localization of hTERT protein is associated with telomerase function. Exp Cell Res 2002;277:201-9.
- Pang JX, Cheng XY, Xu W, Wu SG. Antisense Sp1 oligodeoxy-nucleotide decreases telomerase activity by inhibiting hTERT mRNA expression in Jurkat T cells. Acta Pharmacol Sin 2003;24:91-6.
- Kraemer K, Fuessel S, Schmidt U, Kotzsch M, Schwenzer B, Wirth MP, et al. Antisense-mediated hTERT inhibition specifically reduces the growth of human bladder cancer cells. Clin Cancer Res 2003;3794:3794-800.
- Wang SQ, Lin L, Chen ZB, Lin RX, Chen SH, Guan W, et al. Effect of antisense oligonucleotides targeting telomerase catalytic subunit on tumor cell proliferation in vitro. Chin Sci Bull 2002;47:993-7.
- Nguyen MH, Keeffe EB. Screening for hepatocellular carcinoma. J Clin Gastroenterol 2002;35:s86-91.
- Du QY, Wang XB, Chen XJ, Zhang W, Wang SQ. Antitumor mechanism of antisense Cantide targeting human telomerase reverse transcriptase. World J Gastroenterol 2003;9:2030-5.
- Hu YP, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res 2003;9:2826-36.
- Daniel E, Menezes L, Mayer LD. Pharmacokinetics of Bcl-2 antisense oligonucleotide (G3139) combined with doxorubicin in SCID mice bearing human breast cancer solid tumor xenografts. Cancer Chemother Pharmacol 2002;49:57-68.
- Pastorino F, Brignole C, Marimpietri D, Paolo D, Zancolli M, Pagnan G, et al. Targeted liposomal c-myc antisense oligodeoxy-nucleotides induce apoptosis and inhibit tumor growth and metastases in human melanoma models. Clin Cancer Res 2003;9:4595-605.
- Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M, et al. Alpha-fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carci-noma: a prospective study. Hepatology 2002;35:853-60.
- He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of alpha-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol 1999;5:111-5.
- Konopleva M, Tari AM, Estrov Z, Harris D, Xie Z, Lopez-Berestein G, et al. Liposomal Bcl-2 antisense oligonucleotides enhance proliferation, sensitize acute myeloid leukemia to cytosine-arabinoside, and induce apoptosis independent of other antiapoptotic proteins. Blood 2000;95:3929-39.
- Luger SM, O’Brien SG, Ratajczak J, Ratajczak MZ, Mick R, Stadtmauer EA, et al. Oligodeoxynucleotide-mediated inhibition of c-myb gene expression in autografted bone marrow: a pilot study. Blood 2002;99:1150-8.
- Zellweger T, Chi K, Miyake H, Adomat H, Kiyama S, Skov K, et al. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res 2002;8:3276-84.
- Wang H, Cai Q, Zeng X, Yu D, Agrawal S, Zhang R, et al. Antitumor activity and pharmacokinetics of a mixed-backbone antisense oligonucleotide targeted to the R I alpha subunit of protein kinase A after oral administration. Proc Natl Acad Sci USA 1999;96:13989-94.
- Zhang Y, He DM. Effect of antisense hTERT mRNA oligodeoxy-nucleotide on telomerase activity of leukemic cells. Cell Biol Int 2002;26:427-31.
