Overexpression of dishevelled-1 attenuates wortmannin-induced hyperphosphorylation of cytoskeletal proteins in N2a cell1
Introduction
Abnormally phosphorylated microtubule-associated protein tau is the major protein subunit of paired helical filaments (PHF) in the brain of Alzheimer disease (AD) patients, and accumulation of PHF in affected neurons leads to formation of neurofibrillary tangles (NFT)[1]. In addition, neuro-filament, another neurospecific intermediate filament, is also hyperphosphorylated and accumulated in AD brain[2,3]. Although the precise mechanism for cytoskeleton hyper-phosphorylation is not currently understood, it is widely recognized that an imbalanced regulation in phosphorylation (catalyzed by protein kinases) and dephosphorylation (catalyzed by protein phosphatases) system may play an important role in this pathological process.
Studies have shown that various protein kinases, such as mitogen-activated protein kinases (MAPK), protein kinase A (PKA), cyclin-dependent kinase (CDK), and glycogen synthase kinase-3 (GSK-3), phosphorylate tau at several sites found in AD brain. Among them, GSK-3 is one of the most implicated[4]. Recent studies have shown that tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated in rat brain[5]. GSK-3 is co-localized with hyperphosphorylated tau in degenerating neurons in AD brain[6]. It also phosphorylates neurofilament proteins[7,8]. GSK-3 is a downstream element of phosphatidylinositol-3 kinase (PI-3K), and it is inhibited by protein kinase B (PKB)-catalyzed phosphorylation at Ser-9 of GSK-3β and Ser-21 of GSK-3α. PKB in activity is stimulated by PI-3K-mediated phosphorylation[9]. As there is no direct GSK-3 activator available now, wortmannin is generally used to activate GSK-3 indirectly through inhibiting PI-3K[10] and overactivated GSK-3 phosphorylates tau in rat brain[11,12]. Based on the above information, we used wortmannin to produce a cell model with hyperphosphorylation of cytoskeletal proteins in the present study.
To search for the strategies in arresting Alzheimer-like hyperphosphorylation of cytoskeletal proteins, we used overexpression of dishevelled (DVL) protein. DVL is a cytoplasmic protein involved in the wingless signaling (Wnt) pathways[13]. Studies have shown that Wnt signaling pathways play important roles in AD. Overexpression of DVL-1 protein inhibits GSK-3β-mediated phosphorylation of tau in transfected CHO cells[14] and increases secreted amyloid precursor protein α (sAPPα) production in transfected HEK293 cells[15]. There is no report about the role of DVL-1 on neuro-filament phosphorylation, and the role of DVL-1 on tau phosphorylation in neuronal cells is also not known.
In the present study, we used wortmannin to produce hyperphosphorylation of neurofilament and tau in N2a cells and then determined the effect of mouse DVL-1 protein overexpression on neurofilament and tau hyperphosphoryla-tion.
Materials and methods
Chemicals Polyclonal antibody 111e against total tau, monoclonal antibody PHF-1 against PHF-tau phosphorylated at Ser396/404, monoclonal antibody M4 against PHF-tau phosphorylated at Thr231/Ser235, and monoclonal antibody Tau-1 against PHF-tau unphosphorylated at Ser198/199/202 were gifts from Dr Grundke-Iqbal (New York State Institute for Basic Research, Staten Island, NY, USA), Dr Davies (Albert Einstein College of Medicine, Bronx, NY, USA), and Dr Binder (Northwestern University, Chicago, IL, USA). Monoclonal antibodies SMI31 against phosphorylated neurofilament and SMI32 against unphosphorylated neurofilament were purchased from Sternberger Mono-clonals, Inc (Baltimore, MD, USA). Oregon Green 488-conjugated goat anti-mouse IgG (H+L) was from Molecular Probes (Eugene, OR, USA). Mouse monoclonal antibody c-Myc Ab-2 and wortmannin were purchased from Sigma Chemical Co (St Louis, MO, USA). Bicinchoninic acid (BCA) protein detection kit was obtained from Pierce Chemical Company (Rockford, IL, USA).
Cell culture, plasmid and transfection Mouse neuroblastoma 2a (N2a) cells were obtained from Dr Hua-xi XU (Rockefeller University, NY, USA). The cells were cultured in a medium containing 50% Dulbecco’s modified Eagle’s medium (DMEM) and 50% Opti-MEM, supplemented with 5% fetal bovine serum (Gibico BRL, Gaithersburg, USA) in 5% CO2 at 37 °C.
Mouse DVL-1 subcloned into the ClaI site of pCS2+ in frame with 6-Myc epitope at the C termini was a gift from Dr Lin MEI (University of Alabama at Birmingham, USA). The plasmid and the vector as a control were prepared using a maxi-prep endotoxin-free kit (Qiagen, Crawley, West Sussex, UK) and transfected into N2a cells using LipofectamineTM 2000 (Invitrogen, Carlsbad, California, USA) in a 6-well format. The amounts and volumes were given on a per well basis. One day before transfection, about (2–8)×105 cells were plated in 2 mL of growth medium without antibiotics and the confluence of the cells was about 90%–95% at the time of transfection. For each transfection, 4.0 µg DNA in 250 µL of Opti-MEM medium and 10 µL LipofectamineTM 2000 in 250 µL of Opti-MEM medium were prepared and incubated for 5 min at room temperature. Then, the diluted DNA and the diluted LipofectamineTM 2000 were mixed gently and incubated for another 20 min at room temperature. The mixture was then added to each well and incubated with the cells at 37 °C in a CO2 incubator for 48 h prior to determination of the expression of transfected genes. Then the cells were treated with wortmannin 1 µmol/L[16] and harvested at 0, 1, 3, or 6 h after the treatment.
Western blot Cultures were homogenized in the buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L sodium chloride, 1% NP-40, 0.5% sodium deoxychlolate, 0.1% sodium dodecyl sulfate, 0.02% sodium azide, 0.1 g/L phenyl-methysulfonyl fluoride, and 1 mg/L aprotinin. Protein concentration was determined with BCA Protein Assay Reagent. A middle-molecule weight protein marker was used (Pierce, USA). Equal amounts of protein were separated on either 7.5% or 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech, USA). The membranes were blocked with 3% BSA in TBS at room temperature for 1 h, then incubated with primary antibodies and secondary antibodies, and finally developed using the Enhanced Chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech, USA).
Immunofluorescence microscopy Culture medium was carefully removed. After two rinses in PBS, the cells were fixed in a freshly prepared solution of 4% paraformaldehyde for 1.5 h. After two more rinses in PBS, the cells were perme-abilized in 1% Triton X-100 in PBS for 15 min. Then the cells were incubated in 3% BSA in PBS for 1 h and incubated with primary antibody at 4 °C overnight. After three rinses with PBS, cells were incubated in Oregon Green 488-conjugated secondary antibody (1:1000; Molecular Probes) for 1 h at room temperature. After three more rinses in PBS, fluorescence was observed using a fluorescence microscope (BX60; Olympus, Tokyo, Japan) with appropriate filter sets.
Statistical analysis All data were presented as mean±SD and analyzed by ANOVA followed by Student-Newman-Keuls test to determine the differences among groups.
Results
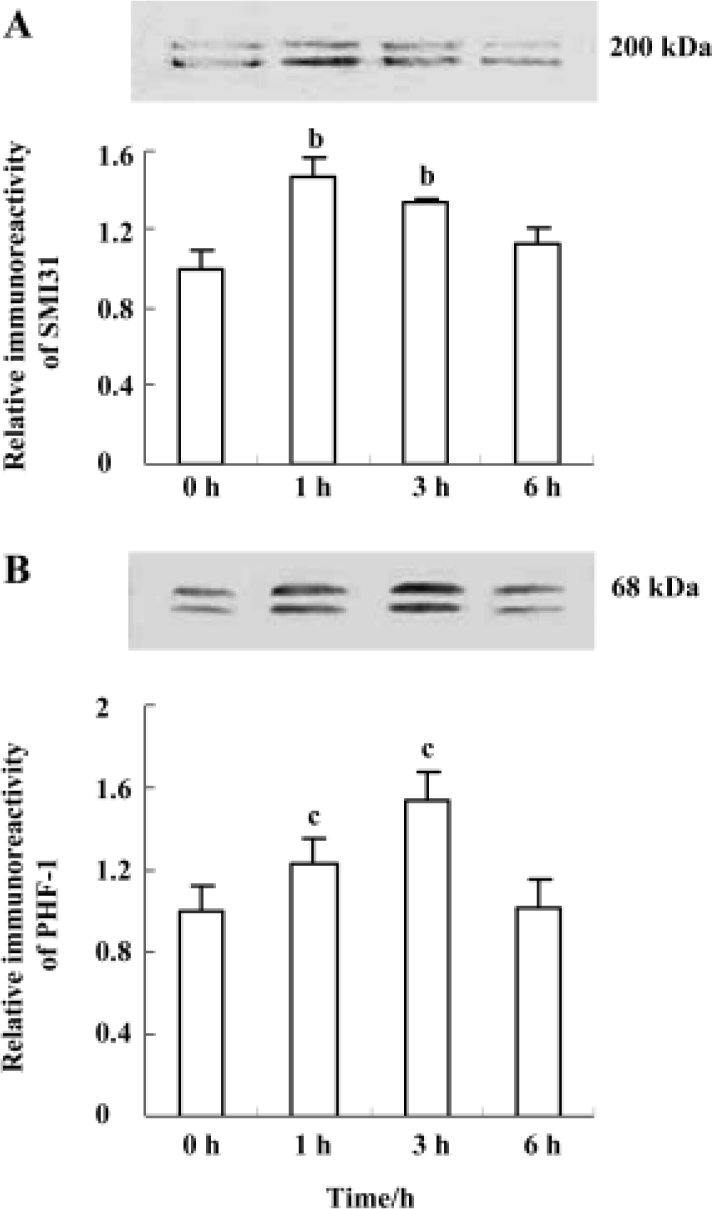
Wortmannin induces transient hyperphosphorylation of neurofilament and tau in N2a cells Phosphorylation of neurofilament and tau in N2a cells were measured by Western blot. The level of phosphorylated neurofilament determined by SMI31 antibody was increased at 1 h and at 3 h, and it was restored to normal level at 6 h (Figure 1A); phosphorylation of tau recognized by PHF-1 antibody was elevated at 1 h, peaked at 3 h, and then back to the normal level at 6 h (Figure 1B) after treatment of the cells with wortmannin (1 µmol/L). The highest level of neurofilament and tau phosphorylation was seen at 1 h and 3 h, respectively (Figure 1). These data demonstrate that wortmannin can induce hyperphosphorylation of neurofilament and tau in N2a cells.
Overexpression of mouse DVL-1 protein inhibits wortmannin-induced neurofilament and tau hyperphos-phorylation in N2a cells As wortmannin induces neurofila-ment and tau hyperphosphorylation most efficiently at 1 h and 3 h, respectively, in N2a cells, we investigated the effect of mouse DVL-1 protein overexpression on neurofilament and tau hyperphosphorylation at these two time points. The enhanced SMI31 (recognizes phosphorylated epitope) and the dimmed SMI32 (recognizes unphosphorylated epitope) immunoreactivity induced by wortmannin were efficiently attenuated when DVL-1 protein was overexpressed (Figure 2A, 2B), suggesting the role of DVL-1 in arresting wort-mannin-induced neurofilament hyperphosphorylation. DVL-1 overexpression also efficiently arrested wortmannin-induced tau hyperphosphorylation at PHF-1 (recognizes phosphorylated epitope, Figure 2C), M4 (recognizes phosphorylated epitope, Figure 2D), and Tau-1 (recognizes unphos-phorylated epitope, Figure 2E) sites. Neither wort-mannin alone nor wortmannin in combination with DVL-1 or with vector changed the level of total tau determined by 111e antibody (Figure 2F). The overexpression of DVL-1 protein was confirmed with anti-c-Myc tag antibody (Figure 2G). Inhibition of wortmannin-induced tau hyperphospho-rylation at both PHF-1 and Tau-1 sites was also observed by immunofluoresce staining of the cells (Figure 3). We also noticed that the immunostaining pattern of Tau-1 antibody was different from that of PHF-1 and 111e and the distribution of Tau-1 staining was more concentrated into the cell membrane, especially in the cells transfected with DVL-1 and treated with wortmannin (Figure 3).


Discussion
Abnormal hyperphosphorylation and accumulation of tau protein in the affected neurons are recognized early pathological processes in AD brain. Several protein kinases are reported to hyperphosphorylate tau at some of the AD epitopes and GSK-3 is one of them[4]. In addition, GSK-3 also phosphorylates neurofilament in vitro[7] and in transfected cells[8]. As there is no direct activator for GSK-3, wortmannin, a specific inhibitor of PI3K[10], has been used to stimulate GSK-3 indirectly to phosphorylate tau in vivo[11,12]. In the present study, we used wortmannin to treat N2a cells and observed that wortmannin induced neurofilament and tau hyperphosphorylation, thus a cell model with Alzheimer-like hyperphosphorylation was established. We also noticed that the number of positive staining bands for tau and neurofilament was different when different antibodies were used. It is known that tau has at least six isoforms by alternative splicing[17]. The number of isoforms of tau and neurofilament shown on the blots depends on the species of the samples, the exposure of epitopes, and the degree of posttranslational modifications, such as phosphorylation.
Though hyperphosphorylation of cytoskeletal proteins plays a critical role in AD pathogenesis, there is no effective tool to arrest the pathological processes. In the present study, we observed that mouse DVL-1 protein overexpres-sion efficiently inhibited wortmannin-induced hyperphos-phorylation of neurofilament at SMI31 and SMI32 epitopes and tau at PHF-1 (Ser-396/404), M4 (Thr-231/Ser-235) and Tau-1 (Ser-198/199/202) epitopes in N2a cells. As wortmannin is an indirect activator of GSK-3, we speculate that DVL-1 may function through arresting the activity of GSK-3. DVL-1 is a component of the wingless pathway (Wnt signaling) and activation of wingless results in inhibition of GSK-3[18] and tau dephosphorylation in hippocampal neurons[19]. In Drosophila imaginal disc cells, overexpression of DVL-1 mimics the wingless signal[20]. These data suggest that loss of function of Wnt signaling may be involved in the pathological process of AD and that inhibition of GSK-3 activity by overexpression of DVL-1 may be useful in antagonizing Alzheimer-like hyperphosphorylation of cytoskeletal proteins. It was also reported that inactivation of GSK-3 induced by wingless may involve activation of PKC[18]. The precise mechanism through which DVL-1 functions is complex and further study is needed to illustrate the underlying mechanisms. Additionally, we also noticed that Tau-1 staining was more concentrated into the cell membrane after DVL-1 transfection and wortmannin treatment in immunocytochemistry study. We currently do not understand the meaning and the mechanism for this translocation of tau.
Taken together, we have found that overexpression of DVL-1 protein effectively attenuates wortmannin-induced neurofilament and tau hyperphosphorylation in N2a cells.
References
- Iqbal K, Grundke-Iqbal I, Smith AJ, George L, Tung YC, Zaidi T. Identification and localization of a tau peptide to paired helical filaments of Alzheimer disease. Proc Natl Acad Sci USA 1989;86:5646-50.
- Wang JZ, Tung YC, Wang YP, Li XT, Iqbal K, Grundke-Iqbal I. Hyperphosphorylation and accumulation neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett 2001;507:81-7.
- Gong CX, Wang JZ, Iqbal K, Grundke-Iqbal I. Inhibition of protein phosphatase 2A induces phosphorylation and accumulation of neurofilaments in metabolically active rat brain slices. Neurosci Lett 2003;340:107-10.
- Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett 1998;436:28-34.
- Liu SJ, Zhang JY, Li HL, Fang ZY, Wang Q, Deng HM, et al. Tau becomes a more favorable substrate for GSK-3 when it is prephos-phorylated by PKA in rat brain. J Biol Chem 2004;279:50078-88.
- Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 1997;56:70-8.
- Guan RJ, Khatra BS, Cohlberg JA. Phosphorylation of bovine neurofilament proteins by protein kinase FA (glycogen synthase kinase 3). J Biol Chem 1991;266:8262-7.
- Guidato S, Tsai LH, Woodgett J, Miller CC. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J Neurochem 1996;66:1698-706.
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785-9.
- Welsh GI, Foulstone EJ, Young SW, Tavare JM, Proud CG. Wortmannin inhibits the effects of insulin and serum on the activities of glycogen synthase linase-3 and mitogen-activated protein kinase. Biochem J 1994;303:15-20.
- Liu SJ, Wang JZ. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol Sin 2002;23:183-7.
- Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, et al. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphos-phorylation of tau and impairment of spatial memory. J Neurochem 2003;87:1333-44.
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev 1994;8:118-30.
- Wagner U, Brownlees J, Irving NG, Lucas FR, Salinas PC, Miller CC. Overexpression of the mouse dishevelled-1 protein inhibits GSK-3β-mediated phosphorylation of tau in transfected mammalian cells. FEBS Lett 1997;411:369-72.
- Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, Pollard C, et al. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J Neurosci 2001;21:4987-95.
- Yu J, Deng YQ, Yang Y, Zhang JY, Zhang YP, Zhang SH, et al. Activation of glycogen synthase kinase 3 induces Alzheimer-like hyperphosphorylation of cytoskeleton protein and cell damage. Prog Biochem Biophys 2004;31:532-7.
- Andreadis A, Broderick JA, Kosik KS. Relative exon affinities and suboptimal splice site signals lead to non-equivalence of two cassette exons. Nucleic Acids Res 1995;23:3585-93.
- Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J 1996;15:4526-36.
- Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res 2004;297:186-96.
- Yanagawa S, Van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev 1995;9:1087-97.
