Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus1
Introduction
Human hepatocellular carcinoma (HCC) has been proven to display high resistance to chemotherapeutic agents, such as cisplatin, 5-fluorouracil (5-FU), and doxorubicin et al[1–3]. This phenomenon is partially due to defects in caspase activation, the execution phase of apoptosis. The inhibitor of apoptosis proteins (IAP) is a family of caspase inhibitors that bind and inhibit the activation of caspases-3, -7, and -9. By inhibiting downstream caspases-3 and -7, IAP block the convergence point of multiple caspase activation pathways and thus inhibit apoptosis from multiple stimuli[4–6]. IAP overexpression, the X-linked inhibitor of apoptosis protein (XIAP) in particular, is associated with chemoresistance in a variety of human cancers[7,8]. Therefore the removal of IAP inhibition could be critical for sensitizing cancer cells to various chemo-anticancer regiments. The second mitochondria-derived activator of caspases (Smac)/Direct IAP Binding Protein with Low PI (DIABLO), an IAP-binding protein, is released from mitochondria and promotes caspase activation by eliminating IAP function during apoptosis[9–11]. Consequently, the overexpression of active Smac could be proposed to render resistant tumor cells sensitive to chemotherapeutic treatment.
ONYX-015, a tumor-targeting oncolytic adenoviral agent that selectively replicates in tumor cells due to E1B-55K gene deletion, has been widely investigated in cancer therapy. This genetic design takes advantage of the fact that adenovirus E1B 55K binds and inactivates the wild-type p53 protein that is essential to virus replication. ONYX-015 has been well tolerated in phase I and II trials, but durable objective responses were not achieved in patients[12,13]. However, durable regressions were subsequently achieved in combination with chemotherapy (such as cisplatin) in head and neck cancer patients[14]. Importantly, ONYX-015 and cisplatin do not have overlapping toxicities[15]. Although the conjugation of ONYX-015 with chemo-agents has attained potent antitumor activity with the reduction of drug resistance and side-effects to some extent, further improvements could be acquired by arming the single viro agent with therapeutic genes.
In this study, we employed a gene-viro agent ZD55-Smac to sensitize HCC cells to chemotherapy (cisplatin or 5-FU). ZD55 was constructed based on adenovirus serotype 5 (Ad5), with E1B-55K gene deletion, but different from ONYX-015 that was designed for the incorporation of therapeutic genes. ZD55-Smac was produced by inserting the Smac gene into the vector, driven by the human cytomegalovirus immediate-early (CMV-IE) promoter and terminated by the simian virus 40 polyadenylation signal. As an oncolytic transgenic delivery system, ZD55 can not only specifically replicate and lyse in tumor cells, but also restrict therapeutic gene expression within a tumor microenviroment[16–18]. Here, we have observed that ZD55-Smac could significantly enhance the sensitivity of HCC cells to both cisplatin and 5-FU. Furthermore, the toxic effects to normal cells are distinctly abolished by utilizing the oncolytic vector and reducing the drug dosage.
Materials and methods
Cell culture The normal human liver cell lines L-02 and QSG-7701, human HCC cell lines BEL7404 (with p53 deletion), SMMC7721, and Huh-7 were purchased from Shanghai Cell Collection (Shanghai, China). HEK293 (a human embryonic kidney cell) was obtained from Microbix Biosystems (Toronto, ON, Canada). The cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco–BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco–BRL, Grand Island, NY, USA) at 37 °C in a humidified incubator with 95% air and 5% CO2.
Generation of recombinant viruses The ZD55 system was constructed by deleting the E1B-55K gene based on adenovirus serotype 5 and introducing a BglII clone site for carrying foreign genes. The Smac gene was then cloned into this site to generate oncolytic adenovirus ZD55-Smac[17]. ONYX-015 and wild-type adenovirus (Ad–wt) were previously stored in our laboratory. The large-scale purification of adenoviruses was performed by ultracentrifugation with cesium chloride. The titers were determined by plaque formation assay on the HEK293 cells.
Conventional PCR for verification The viral genome was purified from the ZD55-Smac recombinant oncolytic adenovirus by using a virus DNA-QIAGEN kit (QIAGEN, Hilden, Germany). The Smac codon sequence (720 bp) was verified by conventional PCR using the following primer (forward: 5'-ATGGCGGCTCTGAAGAGTTGGCTGT-3' and reverse: 5'-TCAATCCTCACGCAGGTAGGCCTCC-3'). The amplification product was visualized by electrophoresis on a 2% agarose gel containing ethidium bromide.
Cytopathic effect assay The HCC cell lines BEL7404 and Huh-7 and normal liver cell lines L-02 and QSG-7701 were cultured in 24-well plates and infected with Ad-wt, ONYX-015, and ZD55-Smac, respectively, at different MOI (Multiplicity of Infection=ratio of infectious virus particles to cells). Four days after infection, the cells were washed, paraformaldehyde-fixed, stained with crystal violet, and scanned on a Bio Imagine System scanner (Syngene, San Diego, CA, USA).
Western blot analysis Total proteins were separated on 8%–12% polyacrylamide gels and transferred onto 0.45 µm nitrocellulose in a buffer containing 25 mmol/L Tris-HCl (pH 8.3)/192 mmol/L glycine/20% methanol and blocked with Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA) for at least 1 h. The membranes were incubated with primary antibodies, detected by the addition of antirabbit infrared (IR) dye 700 or antimouse IR dye 800 (Li-Cor, Lincoln, Nebraska, USA). The fluorescent signal was revealed by using the Odyssey infrared imaging system (Li-Cor, Lincoln, Nebraska, USA). The primary antibodies were from Santa Cruz Biotechnology [Santa Cruz, CA, USA; anti-poly-adenyl ribonuclease polymerase (PARP), anti-E1A, and anti-actin].
In vitro cell viability assay The cells were plated in 96-well plates and treated with ZD55–Smac, cisplatin, 5-FU, or a combination of viruses with drugs. After incubating for the indicated intervals, cell viability was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 0.5 mg/mL) assay.
Apoptotic cell staining Huh-7 HCC cells or L-02 normal liver cells were seeded in 6-well plates. After being treated for 48 h, the cells were incubated with Hoechst 33342 for 2 h and observed under a fluorescence microscope.
Statistics Values are expressed as the mean±SD. The significance of differences was determined using Student’s t-test, with significance set at P<0.05.
Results
Construction and characterization of ZD55-Smac ZD55 is an oncolytic adenovirus, which can selectively replicate and lyse in a great number of tumor cells due to the deletion of the E1B-55K gene from Ad5 that is similar to the typical viro agent ONYX-015. In this vector, we designed a BglII clone site transferring exogenous genes, that is, the main character different from ONYX-015. The Smac gene was inserted into this site, driving by the human CMV-IE promoter.
To determine the selective cytopathic effect (CPE) of ZD55-Smac, 2 HCC cell lines (BEL7404 and Huh-7) and 2 normal liver cell lines (L-02 and QSG-7701) were infected with ZD55-Smac, ONYX-015, or Ad-wt at various MOI. The cells were stained with crystal violet 4 d later. ZD55-Smac performed robust cytotoxity in the HCC cells that was equal or a little higher in comparison with either ONYX-015 or Ad-wt, while a significant suppression of cell proliferation was not observed in normal liver cells (Figure 1).
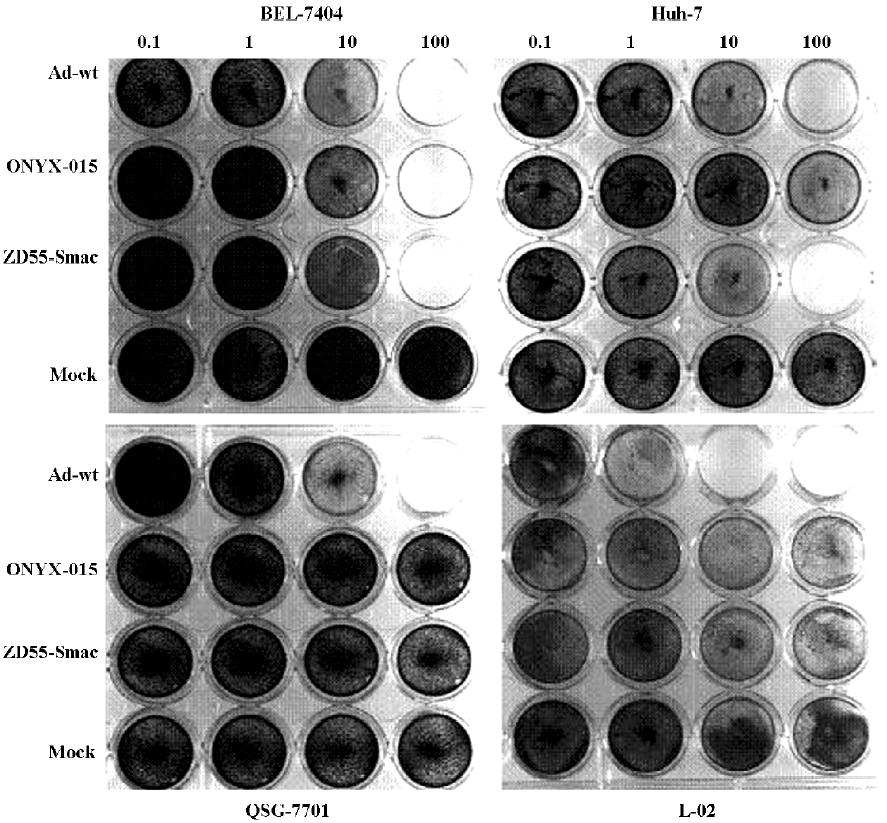
Consequently, the CPE assay indicated that the ZD55 system could specifically induce toxicity in HCC cancer cell lines regarding the tumor selective replication capability, and Smac displayed slight toxicity to Huh-7 cells that was consistent with the results of Figure 2, but do not affect normal cell viability. These observations suggest that ZD55 is an ideal tumor-specific replicative adenovirus for Smac gene delivery.
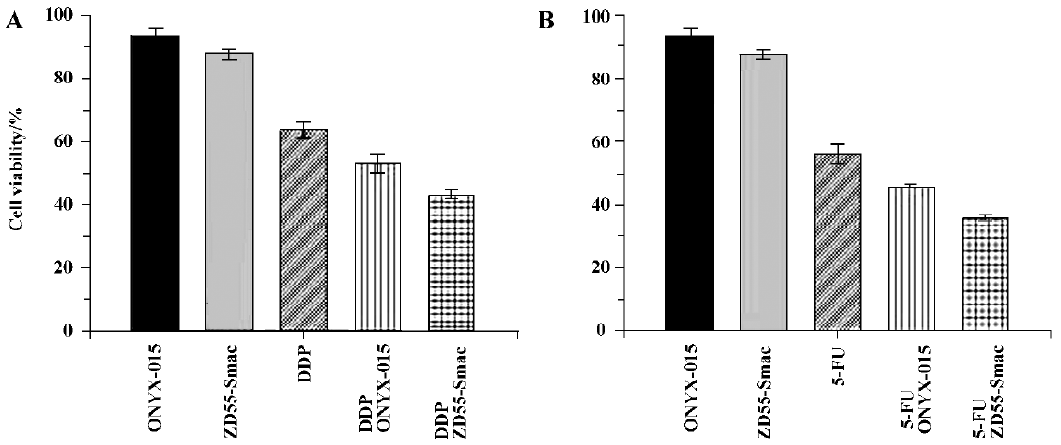
ZD55-Smac is superior to ONYX-015 in sensitizing chemotherapy ONYX-015 has been extensively studied as an effective viro agent for relieving chemoresistance both in experimental and clinical cancer therapy. Here, we proposed to evaluate the assistance capability of ZD55-Smac in enhancing the tumor cytotoxicity of cisplatin- or 5-FU-treated Huh-7 cells compared with ONYX-015. Significant cell growth inhibition was detected in the groups treated with a combination of ZD55-Smac (MOI=1) with cisplatin (1 µg/mL)/5-FU (5 µg/mL), compared with the groups treated with a combination of ONYX-015 (MOI=1) with cisplatin (1 µg/mL)/5-FU (5 µg/mL) (MOI=1), or ZD55-Smac (MOI=1), ONYX-015 (MOI=1), and cisplatin (1 µg/mL)/5-FU (5 µg/mL) alone (P<0.01; Figure 2). These results indicated that blocking the IAP activity by Smac could further improve the enhanced effects of ONYX-015 with cisplatin or 5-FU. Thus, ZD55-Smac-based chemo-gene virotherapy is obviously superior to conventional ONYX-015-based chemo virotherapy.
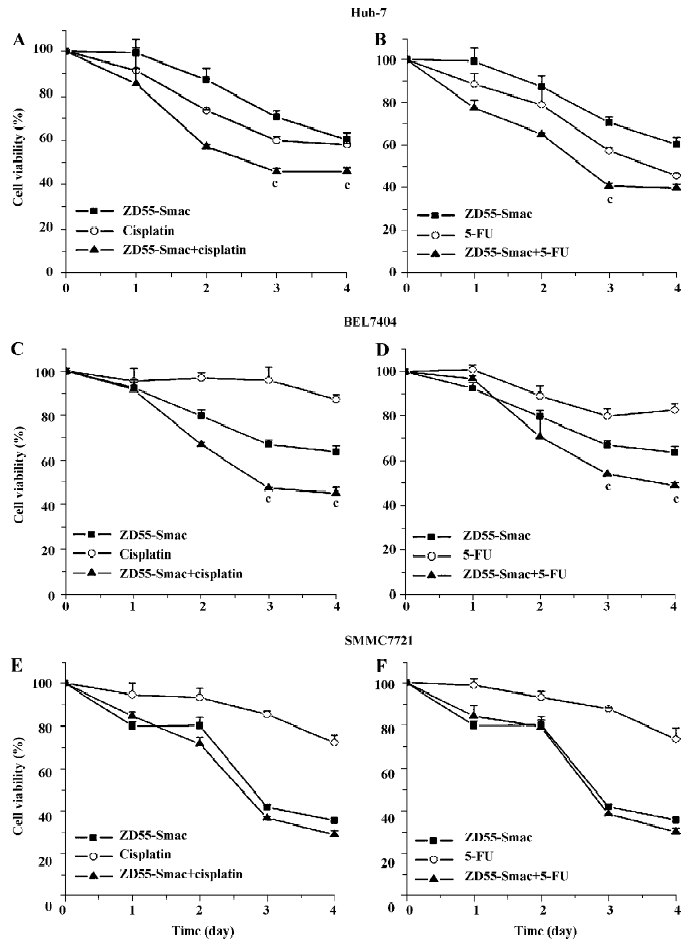
ZD55-Smac enhanced sensitivity of several HCC cell lines to cisplatin or 5-FU To explore the enhanced toxicity of ZD55-Smac with cisplatin or 5-FU, the HCC cells were treated with cisplatin (1 µg/mL), 5-FU (5 µg/mL), ZD55-Smac (MOI=1), and a combination of ZD55-Smac with cisplatin or 5-FU, respectively. According to the results of the MTT assay, a significant decrease in cell viability was observed in the cells treated with ZD55-Smac plus cisplatin or 5-FU (Figure 3). For instance, in the Huh-7 cells, the cell viability significantly decreased 3 d post-treatment in the group of ZD55-Smac plus cisplatin, compared with both the ZD55-Smac alone and cisplatin alone groups (P<0.01; Figure 3). However, the toxic effects are different from the HCC cell types. Using SMMC7721 as an example, ZD55-Smac played an essential role in killing cancer cells, but only promoted the effects of the chemo drugs to a limited extent.
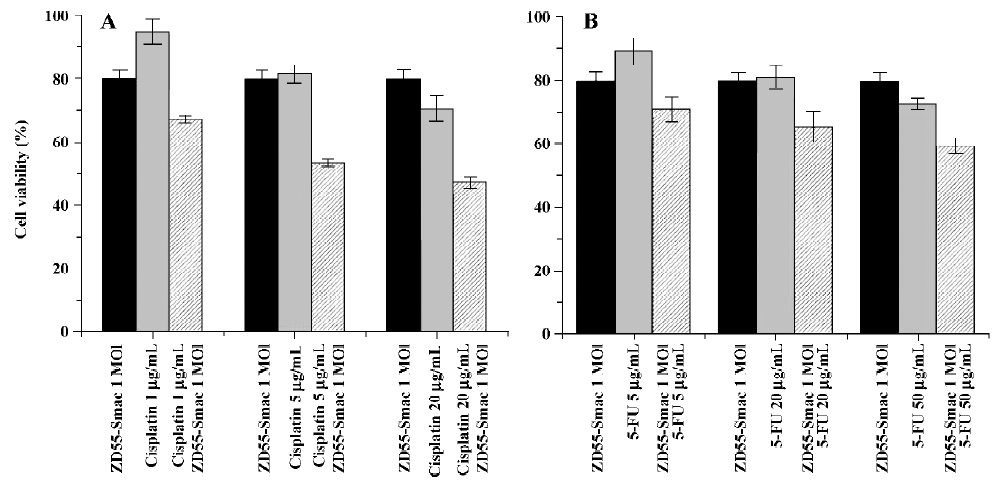
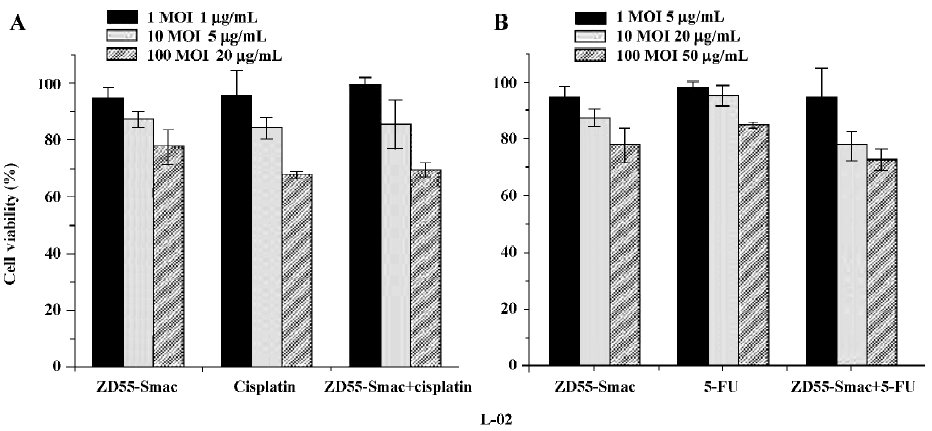
To further evaluate the enhanced cytotoxic effects, a fixed viral titer (ZD55-Smac, MOI=1) was used in combination with variable concentrations of drugs (cisplatin 1, 5, and 20 µg/mL; 5-FU 5, 20, and 50 µg/mL). The cytotoxic effects of the groups using the combination were more apparent compared with either virus or cisplatin/5-FU alone at each concentration (P<0.01; Figure 4).
Although ZD55 is a tumor-selective replication adenoviral vector and the Smac does not have a negative impact on normal cells, extremely high dosages of ZD55–Smac could still cause damage to normal cells. Similarly, the combination of ZD55–Smac with cisplatin or 5-FU also resulted in concentration-dependent cell death as assessed by MTT assay (P<0.01; Figure 5). Importantly, the combination of ZD55-Smac with chemotherapy does not show apparent overlapping toxicities in normal cells.
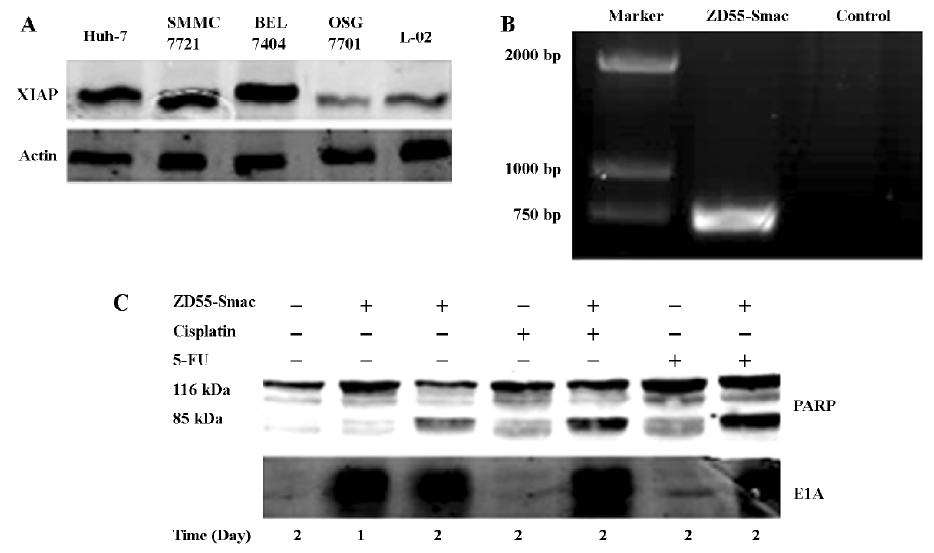
Apoptosis induction by treatment with ZD55-Smac or/and chemotherapy The expression levels of XIAP in HCC cells were analyzed by immunoblot assay. A much higher level of the XIAP protein was detected in Huh-7, BEL7404, and SMMC7721 cells, compared with that in QSG-7701 and L-02 normal liver cells. ZD55-Smac could efficiently mediate Smac expressed in HCC cells as shown in our previous study[17]. Here we verified the virus by conventional PCR and detected adenoviral E1A protein expression in HCC cells by Western blotting (Figure 6). To determine the underlying mechanism by which ZD55-Smac, cisplatin, 5-FU alone, or a combination of ZD55-Smac and cisplatin or 5-FU can induce apoptosis in cancer cells, the activation of PARP in BEL7404 HCC cells was studied by Western blot analysis. The results showed that the cleavage of PARP was more apparent 2 d post-infection with ZD55-Smac, compared with 1 d post-infection for the control group. Furthermore, the combination of ZD55-Smac with either cisplatin or 5-FU led to a slight increase of PARP cleavage, rather than ZD55-Smac, cisplatin, or 5-FU alone (Figure 6).
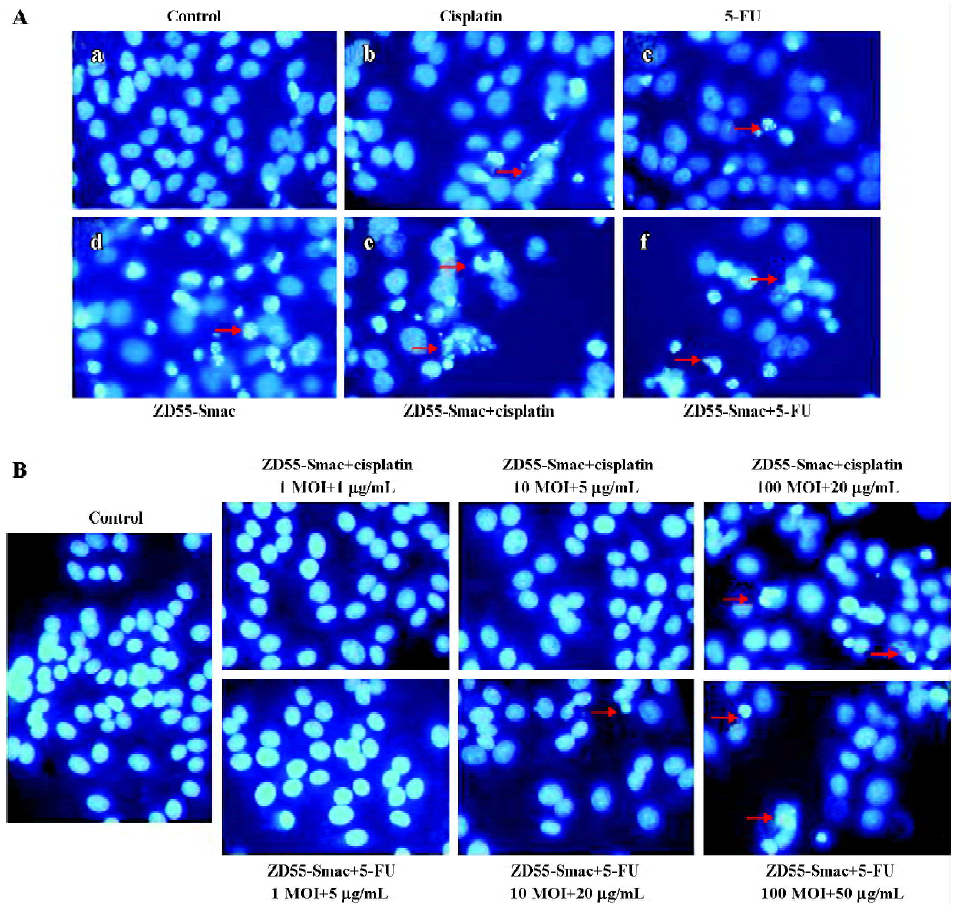
These observations were consistent with the morphological features. Most cancer cells underwent apoptosis during ZD55-Smac treatment in combination with cisplatin or 5-FU as shown by DNA specific fluorochrome staining in Huh-7 cells (Figure 7A). A higher dosage also led to remarkable chromatin condensation and nuclear fragmentation in normal L-02 cells (Figure 7B).
Discussion
Despite the recent introduction of new agents and schedules for the treatment of cancer, chemotherapy still obtains unsatisfactory overall response rates; rare complete remissions; and responses of relatively short duration, mainly due to chemoresistance and severe side-effects. Currently, many studies have revealed the molecular machinery of chemoresistance. Notable advances have been documented in elucidating the mechanisms of drug resistance and sensitization that represent a useful basis for further development of strategies to circumvent chemoresistance in clinical practice.
Apoptosis or programmed cell death is a genetically encoded cell death program characterized by biochemical and morphological changes[19], which has been shown to be the principal mechanism of chemotherapy-induced regression[20]. IAP, including XIAP, cellular inhibitor apoptosis (cIAP) 1 and 2, ronal apoptosis inhibitory protein (NIAP), and survivin, are an evolutionarily conserved family of proteins that prevent cell death by directly inhibiting caspases[21]. XIAP is one of the most potent inhibitors in the family and presents as a potential drug target overexpressed in various human cancer cells[22,23]. In cultured cells, XIAP knock-out directly induced apoptosis and sensitized resistant cells to chemotherapy. In animal models, siRNA or antisense oligonucleotides directed against XIAP delayed tumor growth in human cancer xenografts[24–26]. Moreover, recent studies have shown that Smac can act as an effective pro-apoptotic molecule to bind to IAP[9,10], leading to the design and synthesis of Smac or mimic that can sensitize cancer cells to apoptotic induction by antagonizing IAP[27,28]. Our previous work has also shown great success of attenuating IAP function to enhance the tumor necrosis factor£related apoptosis-inducing ligand (TRAIL)-induced apoptosis by an oncolytic adenovirus (ZD55-Smac) expressing the Smac protein[17]. Cisplatin and 5-FU are widely used anticancer agents with a broad range of antitumor activities. Cisplatin, a platinum-based chemotherapy drug, acts as a factor crosslinking DNA, making it impossible for proper mitosis. The damaged DNA sets off DNA repair mechanisms, which finally activate apoptosis. Cisplatin has been used in many cancers, especially in testicular cancer and epidermal carcinomas[29]. In the case of HCC, cisplatin has been shown to be more effective than other agents and more so in combination with drugs, such as 5-FU, which induces synergistic effects[30,31]. 5-FU is one of the first-line treatment options for gastrointestinal tumors, but its effects on HCC have been negative[32].
In the present study, we utilized ZD55-Smac to sensitize HCC cells to chemotherapeutic agents cisplatin or 5-FU. Our data indicated that the combination of ZD55-Smac and chemotherapy demonstrated robust cytotoxicity, compared with ZD55-Smac, cisplatin, or 5-FU alone as shown in both the MTT assay and cell apoptotic staining. Thus, the dosage could be reduced greatly in order to protect normal cells, according to the increased tumor killing effect within the cooperation of chemo agents and ZD55-Smac. In this study, as shown by the MTT assay in L-02 normal cells, we introduced the lowest dose (MOI=1, ZD55-Smac+1 µg/mL cisplatin or 5 µg/mL 5-FU) to treat cells. These results showed that there was slight damage to the normal cells at such a low dose.
Furthermore, ZD55 is similar to ONYX-015 with E1B-55K gene deletion that can selectively replicate and lyse in a number of cancer cells approved by our previous tests[17,18]. For example, in the case of ONYX-015, which works synergistically with chemotherapy in certain human cancers, we employed ZD55 to deliver Smac and then combined it with chemo agents to treat the HCC cells. Therefore, ZD55 has played triple roles: (1) as an oncolytic viro agent; (2) a transgenic vector; and (3) a chemotherapeutic assistant. As shown in Figure 2, ZD55–Smac was much more efficient in enhancing the sensitivity of HCC cells to chemotherapy than ONYX-015.
In conclusion, the ZD55 adenoviral vector could efficiently transfer Smac into HCC cells. ZD55-Smac conjugated with cisplatin or 5-FU performs enhanced sensitivity of several HCC cell lines to chemotherapy, while evidently relieving the negative toxicity in normal cells by applying the tumor-selective replication vector and reducing the dosage. Moreover, the current chemo-gene virotherapeutic (cisplatin or 5-FU+ZD55-Smac) strategy seems superior to the conventional chemo-gene or chemo-viro approach.
References
- Nishiyama M, Yamamoto W, Park JS, Okamoto R, Hanaoka H, Takano H, et al. Low-dose cisplatin and 5-fluorouracil in combination can repress increased gene expression of cellular resistance determinants to themselves. Clin Cancer Res 1999;5:2620-8.
- Shimada M, Takenaka K, Kawahara N, Yamamoto K, Shirabe K, Maehara Y, et al. Chemosensitivity in primary liver cancers: evaluation of the correlation between chemosensitivity and clinicopathological factors. Hepatogastroenterology 1996;43:1159-64.
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30.
- Deveraux QL, Reed JC. IAP family proteins — suppressors of apoptosis. Genes Dev 1999;13:239-52.
- Chai J, Shiozaki E, Srinivasula SM, Wu Q, Dataa P, Alnemri ES, et al. Structural basis of caspase-7 inhibition by XIAP. Cell 2001;104:769-80.
- Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem 2001; 276: 27 058–63.
- Nakagawa Y, Abe S, Kurata M, Hasegawa M, Yamamoto K, Inoue M, et al. IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am J Hematol 2006;81:824-31.
- Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep 2005;14:993-7.
- Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature 2000;408:1008-12.
- Srinivasula S, Hegde R, Saleh A. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 2001;410:112-6.
- Chai J, Du C, Wu J, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 2000;406:855-62.
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther 2004;10:958-66.
- Kirn D, Hermiston T, McCormick F. ONYX-015. Clinical data are encouraging. Nat Med 1998;4:1341-2.
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med 2000;6:879-85.
- Heise C, Sampson-Johannes A, Williams A, McCormick F, von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med 1997;3:639-45.
- Wang J, Gu JF, Yang SY, Xiao T, Qi R, Sun LY, et al. Security of dual cancer-specific targeting vector and its cytotoxic effect when harbored. Ai Zheng 2006;25:385-92.
- Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, et al. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology 2004;39:1371-81.
- Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther 2005;16:845-58.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239-57.
- Fisher DE. Apoptosis in cancer therapy: Crossing the threshold. Cell 1994;78:539-42.
- Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J 1997;16:6914-25.
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 1997;388:300-4.
- Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 1999;18:5242-51.
- Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res 2003;9:2826-36.
- Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, et al. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res 2003;63:7724-32.
- Chen J, Xiao XQ, Deng CM, Su XS, Li GY. Downregulation of XIAP expression by small interfering RNA inhibits cellular viability and increases chemosensitivity to methotrexate in human hepatoma cell line HepG2. J Chemother 2006;18:525-31.
- Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, et al. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol 2006;1:525-33.
- Galluzzi L, Larochette N, Zamzami N, Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene 2006;25:4812-30.
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005;4:307-20.
- Okamura M, Hashimoto K, Shimada J, Sakagami H. Apoptosis-inducing activity of cisplatin (CDDP) against human hepatoma and oral squamous cell carcinoma cell lines. Anticancer Res 2004;24:655-61.
- Tanioka H, Tsuji A, Morita S, Horimi T, Takamatsu M, Shirasaka T, et al. Combination chemotherapy with continuous 5-fluorouracil and low-dose cisplatin infusion for advanced hepatocellular carcinoma. Anticancer Res 2003;23:1891-7.
- Li YX, Lin ZB, Tan HR. Wild type p53 increased chemosensitivity of drug-resistant human hepatocellular carcinoma Bel7402/5-FU cells. Acta Pharmacol Sin 2004;25:76-82.
