Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation1
Introduction
There is an increasing demand for natural compounds that improve humans’ health. Many nutritive and non-nutritive phytochemicals with diversified pharmacological properties have shown promising responses for the prevention and/or intervention of various cancers[1]. Aloe-emodin (1,8-dihydroxy-3-hydroxymethyl-9,10-anthracenedione) is a herbal anthracenedione derivative from Rhei rhizoma, a traditional Oriental herb commonly used in laxation, antivirus, and hepatoprotection practice[2–4]. Recent reports have shown that aloe-emodin possesses antiproliferation effects on some types of cancer cells, such as lung squamous, glioma, and neuroectodermal cancer cells[5–7]. The anticancer mechanisms of aloe-emodin involve the induction of caspase-dependent apoptosis, where the greater sensitivity of neuroectodermal tumor cell lines could be related to an energy-dependent pathway[5,7]. Furthermore, the antiglioma action of aloe-emodin involves extracellular signal-regulated kinases (ERK) 1 and 2-independent induction of both apoptosis and auto-phagy, as well as the ERK inhibition-mediated differentiation of glioma cells[6]. The inhibitory effect of aloe-emodin on the activity and gene expression of N-acetyltransferase, which plays an initial role in the metabolism of arylamine carcinogens, was found in human malignant melanoma cells[8]. Recently, Lin et al found that aloe-emodin-induced apoptosis in T24 human bladder cancer cells was mediated through the activation of p53, p21, Fas/Apo-1, Bax, and caspase-3[9]. However, the anticancer molecular mechanisms of aloe-emodin are largely unclear, especially for cervical cancer cells.
Cervical cancer continues to be a major public health problem in the world. Of all neoplasms found in females around the world, cervical cancer has the third highest incidence and is the number four cause of death[10,11]. In this study, in order to probe the mechanisms underlying the chemopreventive potential of aloe-emodin on cervical cancer, its effects on cell growth, cell cycle, alkaline phosphatase (ALP) activity, protein kinase C (PKC), and c-myc expressions in HeLa cells were investigated. The results of the present study demonstrated the ability and detail mechanisms of aloe-emodin with the potential anticancer therapeutic activity of cervical cancer.
Materials and methods
Materials and cell line Aloe-emodin (N
Cell culture The HeLa cells were cultured in plastic flasks or multi-well plates at 37 oC in a humidified atmosphere of 5% CO2 with RPMI-1640 medium containing 10% fetal calf serum, 50 000 U/L penicillin, and 50 mg/L streptomycin. The medium was changed every other day. Exponentially growing cells were used in the experiments. For the quantitative assays of proliferation, 1×104 cells were seeded in 96-well plates in regular growth medium and incubated for 24 h. The cells were then incubated in medium at different concentrations of aloe-emodin dissolved in dimethylsulphoxide (Me2SO). The concentrations of aloe-emodin used were 2.5, 5, 10, 20, and 40 µmol/L, respectively. The cells were then treated for 1–5 d and monitored for cell growth using the MTT assay. In all the assays, the vehicle (Me2SO) was present at less than 0.1% and the controls with the vehicle (0.1% Me2SO) were carried out in parallel.
MTT assay Sets of 12 wells were used for each dose in this assay. In total, 30 µL MTT solution [2 g/L in phosphate-buffered saline (PBS)] was added into each of the 96 wells. After the cells were incubated at 37 oC for 4 h, the medium was removed and 150 µL of Me2SO was added to solubilize the formazan. The microplate was shaken on a rotary platform for 10 min. Finally, the optical density (OD) values were measured at 550 nm using a Wellscan reader (Labsystems, Santa Fe, NM, USA). The inhibitive rate was used to indicate the suppressive effect of aloe-emodin on the HeLa cells. Growth inhibition was calculated as a percentage as follows: ([ODcontrol–ODexperiment]/ODcontrol)×100%[12].
Crystal violet assay The intensity of crystal violet staining is directly proportional to the number of adherent cells[13]. In order to observe the long-term antiproliferation effect, the HeLa cells were seeded in flat-bottom 6-well plates at 1×104 cells/well and treated with various concentrations of aloe-emodin for 8 d. Then the medium was removed very carefully by mild suction, and 2 mL/well of 1% glutaraldehyde solution in PBS was added. The plates were incubated for 15 min at room temperature to fix the cells. The fixative was removed and replaced by the same amount of PBS. The PBS was removed and the same amount of 0.02% aqueous solution of crystal violet was added. After incubation at room temperature for 30 min, the crystal violet solution was poured and the plates were washed gently with water. Then 2 mL 70% ethanol was used to release crystal violet. Finally, the absorbance was measured at 570 nm using a Wellscan reader.
Cell cycle analysis and apoptosis measurement A total of 1×106 HeLa cells were treated with various concentrations of aloe-emodin for 1–5 d. The cells were harvested with 0.25% trypsin and sedimented by centrifugation at 937×g for 5 min at room temperature. After the supernatant was removed, ice-cold 70% ethanol was added. Finally, the cell cycle was analyzed with a Coulter flow cytometer (Beckman Coulter, Miami, FL, USA). The cell cycle distribution was estimated according to standard procedures[14]. The percentage of cells in the different cell cycle phases (G0/G1, S, or G2/M phase) was calculated using Coulter Epicx XL-MCL DNA analysis software (Beckman Coulter, USA). The sub-G1 peak was considered a measure of apoptosis[15,16].
Determination of relative ALP activities The HeLa cells were seeded at a density of 1×104 and treated with 2.5, 5, 10, 20, and 40 µmol/L aloe-emodin for 1–5 d before being assayed for ALP activity. The cells were then dissolved with 0.25% sodium deoxycholate. Finally, the ALP activities were measured by dynamics assay with a Screen Master 3000 semi-automatic biochemistry analyzer (Hospitex Diagnostics, Florence, Italy). The relative enzyme activity was expressed as U/g[17,18]. Six wells of a 12-well plate were used for each dose and treatment time. Three independent experiments were performed in this analysis.
Western blotting Total cell lysates from 2×105 cells were prepared by lysing the washed cell pellet directly in radioimmunoprecipitation buffer. The lysates were clarified by centrifugation at 13 000×g for 15 min at 4 °C. The lysates were boiled and separated by SDS–PAGE in 10% polyacrylamide gels, blotted onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA) and analyzed by Western blotting with the antibodies from Boster Bioengineering (Wuhan, China).
Statistics The statistical analysis was performed using SPSS version 10.0 (SPSS, Chicago, IL, USA). The Student’s t-test was used to make a statistical comparison between the groups. The level of significance was set at P<0.05.
Results
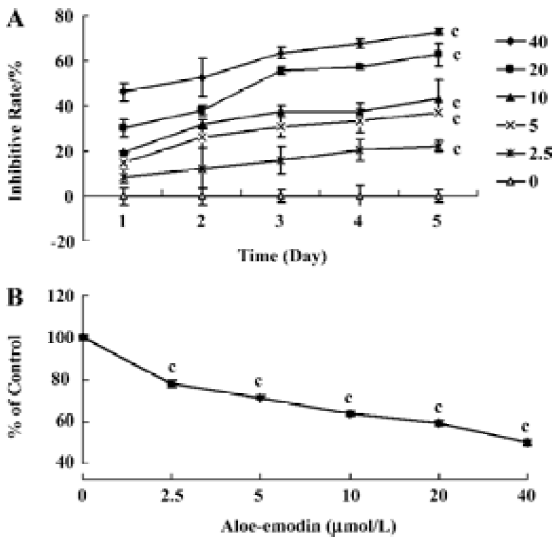
Growth inhibitory effect of aloe-emodin on human cervical cancer cells The effect of aloe-emodin on cell growth was evaluated by MTT assay (Figure 1A). Aloe-emodin inhibited the growth of HeLa cells in a time- and dose-dependent inhibitory manner at concentrations of 2.5–40 µmol/L (at 2.5 µmol/L–20 µmol/L, P<0.01; at 40 µmol/L, P<0.001). Next, to observe the long-term effect, the crystal violet assay was used. The results showed that the number of adherent cancer cells was decreased by aloe-emodin (P<0.001, Figure 1B). These data imply that aloe-emodin has a significant growth inhibitory effect on HeLa cells in vitro.
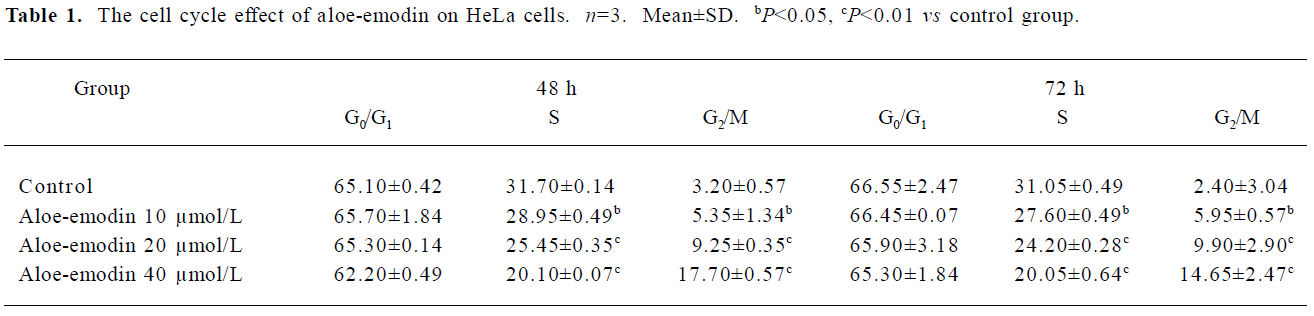
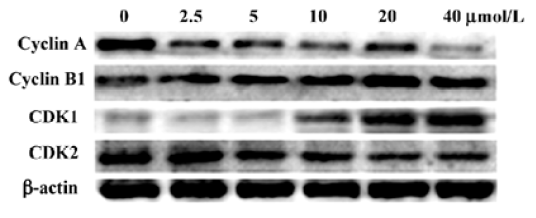
Effect of aloe-emodin on the cell cycle progression of HeLa cells In order to decipher the suppressive mechanisms of aloe-emodin on HeLa cells, changes in the cell cycle distribution were monitored by flow cytometry. The treatment of aloe-emodin resulted in a time-dependent increase in the distribution of cells at the G2/M phase (Table 1). Furthermore, the sub-G1 peak (apoptosis peak) was not observed (data not shown). By using DNA fragmentation analysis, the DNA ladder was not obviously observed (data not shown). Next, the levels of the cell cycle-associated proteins were determined by Western blotting. As shown in Figure 2, aloe-emodin decreased the abundance of cyclin A and cyclin-dependent kinase (CDK) 2, while increased cyclin B1 and CDK1 in the protein levels in a dose-dependent manner. Such data were consistent with arresting cells in the G2/M boundary. Cyclins function as regulators of CDK. Cylin A binds and activates CDK2, and thus promotes both cell cycle G1/S and G2/M transitions[19]. Cyclin B1 binds and activates CDK1, and is expressed predominantly during the G2/M phase[20]. The results obtained from this study suggest that one of the mechanisms of the growth inhibitory effect of aloe-emodin on HeLa cells is through cell cycle arrest, but not by apoptosis induction, at least at the concentrations observed.
Full table
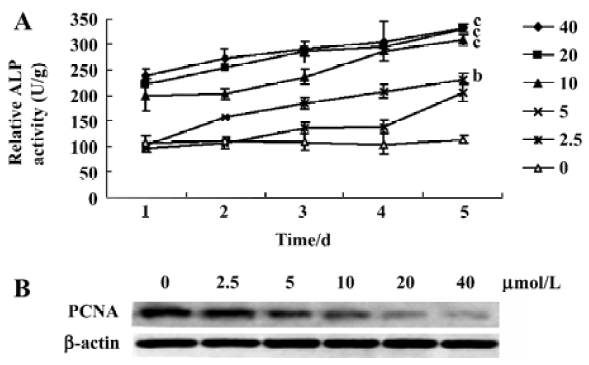
Increase of ALP activity by aloe-emodin In order to determine whether aloe-emodin is capable of affecting ALP activity in HeLa cells, a dynamics assay was used. The ALP activity was increased by aloe-emodin and reached its peak level at 5 d (Figure 3A). The statistical significance between the ALP of the control and treated cells was observed (5 µmol/L, P<0.05; 10, 20, and 40 µmol/L, P<0.01). As the proliferating cell nuclear antigen (PCNA) is a commonly used marker of both cell proliferation and differentiation[21], its changes in the protein level was studied by Western blotting. As shown in Figure 3B, aloe-emodin decreased the abundance of PCNA.
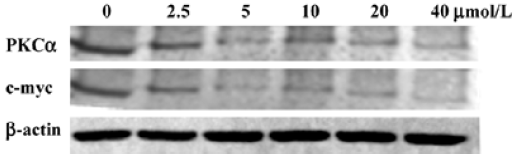
Aloe-emodin decreases PKCα and c-myc in HeLa cells As both the PKC pathway and c-myc participate in a wide range of cellular programs controlling proliferation, differentiation, and survival, the influence of aloe-emodin on the activation of these important signaling molecules in HeLa cells was examined. Western blotting results showed that the PKCα and c-myc protein levels in the control group was high, while after treatment with aloe-emodin, both decreased (Figure 4). A clear dose-dependent decrease was found in cancer cells treated by aloe-emodin (Figure 4).
Discussion
Phytochemicals present in medicinal herbs and dietary plants are one of the most attractive approaches in cancer chemotherapy. Aloe-emodin, a hydroxyanthraquinone from Rhei rhizoma leaves, has been found to have anticancer effects in several cancer cell lines[5–7]. More importantly, aloe-emodin was found to have no appreciable toxic effects in vivo[7]. Until now, the effect of aloe-emodin on cervical cancer has been largely unknown.
The results of the present study clearly demonstrate the anticancer activity of aloe-emodin on human cervical cancer HeLa cells. Aloe-emodin inhibited the growth of HeLa cells in a dose-dependent manner from 2.5 µmol/L to 40 µmol/L (Figure 1A). At the same time, crystal violet assays indicated that aloe-emodin has a durable growth inhibition on cervical cancer cells (Figure 1B).
To examine the mechanism responsible for cell growth inhibition, cell cycle distribution was evaluated using flow cytometry. The loss of the proliferative capacity of cervical cancer cells treated by aloe-emodin was associated with the G2/M phase arrest (Table 1). At the same time, the cell cycle-associated proteins were obviously involved (Figure 2). Similar to our observations, several other studies found that aloe-emodin blocked human glioma U251 cells and promyelocytic leukemia HL60 cells at the G2/M phase[6,22], and hepatoma cells at the G0/G1 phase[23]. These suggest that multiple mechanisms may be responsible for the anticancer effects of aloe-emodin on different types of cancers.
Cellular ALP are increasingly recognized as important markers for monitoring tumor cell behavior in human malignancies. The measurement of ALP activity is usually used to determinate the effect of inducing the differentiation of anticancer reagents[24]. Early in vitro studies showed that some drugs, such as Ara C, peptichemio, or hydrocortisone, inhibited HeLa cell growth with the elevation of ALP activity[25]. In this study, the ALP activity in HeLa cells treated by aloe-emodin increased in a time-and dose-dependent manner (Figure 3A). This is one of the first studies to focus on the expression of ALP in human cervical carcinomas cells treated by aloe-emodin.
Cell signal pathways have been become the targets for many drugs. Among them, the PKC pathway is gaining more and more attention for cancer chemotherapy. Several studies have shown that PKCα plays a role in tumor proliferation and survival[26,27]. In this study, we found that PKCα was suppressed by aloe-emodin (Figure 4). These data strongly suggest that PKCα is one of the key targets of the antitumor action of aloe-emodin. To test this hypothesis, we observed the expression change of c-myc, a target gene of PKCα[28]. As shown in Figure 4, c-myc was also decreased by aloe-emodin. As we know, c-myc is an immediate early gene encoding transcription factors expressed in the G1 phase of the cell cycle and has a DNA-binding property. Furthermore, the inhibition of c-myc by antisense oligomers has been shown to inhibit cell proliferation[29]. From this experiment, it is well established that aloe-emodin has a downregulatory effect on the expression of c-myc in human cervical cancer cells (Figure 4). Since c-myc is the downstream target of the PKC pathway, this effect may be through the PKCα pathway.
Taken together, aloe-emodin can affect cell growth, cell cycle, and ALP activity of human cervical cancer cells in vitro.
References
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3:768-80.
- Krumbiegel G, Schulz HU. Rhein and aloe-emodin kinetics from senna laxatives in man. Pharmacology 1993;47 Suppl 1:120-4.
- Andersen DO, Weber ND, Wood SG, Hughes BG, Murray BK, North JA. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Res 1991;16:185-96.
- Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, et al. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol 2000;87:229-33.
- Lee HZ, Hsu SL, Liu MC, Wu CH. Effects and mechanisms of aloe-emodin on cell death in human lung squamous cell carcinoma. Eur J Pharmacol 2001;431:287-95.
- Mijatovic S, Maksimovic-Ivanic D, Radovic J, Miljkovic Dj, Harhaji Lj, Vuckvic O, et al. Anti-glioma action of aloe emodin: the role of ERK inhibition. Cell Mol Life Sci 2005;62:589-98.
- Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggioni A, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res 2000;60:2800-4.
- Lin SY, Yang JH, Hsia TC, Lee JH, Chiu TH, Wei YH, et al. Effect of inhibition of aloe-emodin on N-acetyltransferase activity and gene expression in human malignant melanoma cells (A375.S2). Melanoma Res 2005;15:489-94.
- Lin JG, Chen GW, Li TM, Chouh ST, Tan TW, Chung JG. Aloe-emodin induces apoptosis in T24 human bladder cancer cells through the p53 dependent apoptotic pathway. J Urol 2006;175:343-7.
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 1999;80:827-41.
- Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999;83:18-29.
- Guo JM, Xiao BX, Dai DJ, Liu Q, Ma HH. Effects of daidzein on estrogen-receptor-positive and negative pancreatic cancer cells in vitro. World J Gastroenterol 2004;10:860-3.
- Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods 1984;68:167-75.
- Liu HS, Chen CY, Lee CH, Chou YI. Selective activation of oncogenic Ha-ras-induced apoptosis in NIH/3T3 cells. Br J Cancer 1998;77:1777-89.
- Patel V, Senderowicz AM, Pinto D Jr, Igishi T, Taffeld M, Quintanilla-Martinez L, et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, suppresses the growth of head and neck squamous cell carcinomas by inducing apoptosis. J Clin Invest 1998;102:1674-81.
- Su SJ, Yeh TM, Lei HY, Chow NH. The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin Cancer Res 2000;6:230-6.
- Herz F, Halwer M. Differential effects of sodium butyrate and hyperosolality on the modulation of alkaline phosphatases of LoVo cells. Exp Cell Res 1990;188:50-4.
- Honma Y, Takenaga K, Kasukabe T, Hozumi M. Induction of differentiation of cultured human promyelocytic leukemia cells by retinoids. Biochem Biophys Res Commun 1980;95:507-12.
- Mendoza N, Fong S, Marsters J, Koeppen H, Schwall R, Wickramasinghe D. Selective cyclin-dependent kinase 2/cyclin A antagonists that differ from ATP site inhibitors block tumor growth. Cancer Res 2003;63:1020-4.
- Jiang H, Luo S, Li H. Cdk5 activator-binding protein C53 regulates apoptosis induced by genotoxic stress via modulating the G2/M DNA damage checkpoint. J Biol Chem 2005; 280: 20 651–9.
- Dworakowska D, Gozdz S, Jassem E, Badzio A, Kobierska G, Urbaniak A, et al. Prognostic relevance of proliferating cell nuclear antigen and p53 expression in non-small cell lung cancer. Lung Cancer 2002;35:35-41.
- Chen HC, Hsieh WT, Chang WC, Chung JG. Aloe-emodin induced in vitro G2/M arrest of cell cycle in human promyelocytic leukemia HL-60 cells. Food Chem Toxicol 2004;42:1251-7.
- Kuo PL, Lin TC, Lin CC. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci 2002;71:1879-92.
- Siddiqui B, Kim YS. Effects of sodium butyrate, dimethyl sulfoxide, and retinoic acid on glycolipids of human rectal adenocarcinoma cells. Cancer Res 1984;44:1648-52.
- D’Ancona S, Barberio MT, Adami P, Poltronieri R. Effect of peptichemio and of peptichemio plus hydrocortisone on growth and alkaline phosphatase activity in HeLa cells in vitro. Boll Ist Sieroter Milan 1980;59:60-9.
- Shen L, Glazer RI. Induction of apoptosis in glioblastoma cells by inhibition of protein kinase C and its association with the rapid accumulation of p53 and induction of the insulin-like growth factor-1-binding protein-3. Biochem Pharmacol 1998;55:1711-9.
- Ahmad S, Mineta T, Martuza RL, Glazer RI. Antisense expression of protein kinase C alpha inhibits the growth and tumorigenicity of human glioblastoma cells. Neurosurgery 1994;35:904-9.
- Zeng X, Xu H, Park BK, Glazer RI. Transformation of mammary epithelial cells by 3-phosphoinositide-dependent kinase-1 (PDK1) is associated with the induction of protein kinase Cα. Cancer Res 2002;62:3538-42.
- Heikkila R, Schwab G, Wickstrom E, Loke SL, Pluznik DH, Watt R, et al. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature 1987;328:445-9.
