Selective enrichment of hepatocytes from mouse embryonic stem cells with a culture system containing cholestatic serum1
Introduction
Embryonic stem (ES) cells are continuously growing cell lines initially isolated from the inner cell mass of blastocysts[1]. Under certain experimental conditions, ES cells spontaneously differentiate into cell types from all 3 germ layers in vitro. Due to the unlimited proliferating ability and pluripotent differentiation potential, ES cells may provide an alternative cell source for cell replacement therapy in the treatment of various diseases. To date, attempts have been made to direct the differentiation of ES cells into a variety of cell lineages in vitro. When cultured in vitro under certain conditions, ES cells can differentiate into cardiac[2–4], neuronal[5–7], hematopoietic[8,9], vascular smooth muscle[10], adipogenic[11], or chondrogenic cell types[12].
Thus far, studies on ES cell differentiation into hepatocytes have mainly focused on genetic and protein analyses of specific markers implicated in the development of the liver[13]. However, little attention has been paid to how to determine and improve the hepatic differentiation ratio. An efficient hepatic differentiation system is very important for subsequent research on hepatocyte transplantation and liver engineering. According to the principle that cells in culture survive when they are adapted to the existing microenviron-ment, we developed an efficient culture system to enrich hepatocyte-like cells from differentiated ES cells. In such a system, only hepatocyte-like cells survive, while other cells do not. This particular culture system contains factors that selectively activate the proliferation of hepatocyte-like cells, but inhibit the growth of other cells. Studies on related mechanisms of liver regeneration and hepatic stem cells corresponding to liver injury have suggested that the pathological serum obtained from patients or animals with severe liver injury may provide such conditions[14–17]. Therefore, in the present study, we utilized a culture system containing cholestatic serum to enrich hepatocyte-like cells from mouse ES-derived differentiated cells in vitro. Our results showed that cholestatic serum is efficient in lineage differentiation and proliferation of hepatocyte-like cells from mouse ES cells, and the resulting hepatocyte-like cells are capable of accumulating glycogen, an important metabolic function of hepatocytes.
Materials and methods
Preparation of the conditional selective medium Cholestatic serum was prepared according to our previously reported method[18]. Sprague-Dawley rats weighing 200–250 g (Laboratory Animal Research Center of Sun Yat-sen University, Guangzhou, China) underwent ligation and transection of the common bile duct under general anesthesia with ether to induce cholestasis. Ten days after the operation, the rats were sacrificed and whole blood was collected. Serum was isolated from the whole blood and then subjected to liver function testing, inactivated, and sterilized for use in culture. Different doses of cholestatic serum were added into the differentiating medium to achieve the following final concentrations: 20, 50, and 100 mL/L. Differentiating medium containing cholestatic serum served as the conditional selective medium in the course of cell differen-tiation. All animal experimental procedures were approved by the Sun Yat-sen University Institutional Animal Care and Use Committee.
Cell culture Undifferentiated E14 mouse ES cells (ATCC, Manassas, VA, USA) were maintained on gelatin-coated dishes in Dulbecco’s modified Eagle’s medium (DMEM;GIBCO, Grand Island, NY, USA), supplemented with 15% fetal bovine serum (Hyclone, Rockville, MD, USA), 1000 U/mL recombinant mouse leukemia inhibitory factor (LIF;Chemicon, Temecula, CA, USA), 1% non-essential amino acids, 1 mmol/L glutamine, 0.1 mmol/L β-mercaptoethanol (Sigma, St Louis, MO, USA), and 10 ng/mL recombinant human fibroblast growth factor-4 (FGF-4; R&D, Minnea-polis, MN, USA). The above culture medium, excluding LIF, was defined as the differentiating medium.
To induce differentiation, the ES cells were incubated using the suspension culture methods described previously[19–21]. Briefly, the cells were suspended in the differentiating medium, designated as d 0 of cell differentiation, and allowed to develop into embryoid bodies (EB). After 4 d in the culture, the resulting EB were plated onto 6-well plates coated with 0.1% gelatin, and cultured with the differentiating medium in the presence of 3 mmol/L sodium butyrate. One week later, to isolate and enrich the ES-derived hepatocyte-like cells from mixed differentiated cells, the cells were further cultured in the conditional selective medium supplemented with 3 mmol/L sodium butyrate and 10 ng/mL recombinant mouse hepatocyte growth factor (HGF; R&D, USA). Under these conditions, differentiated cells were cultured for another 10 d. The cells cultured in the differentiating medium without sodium butyrate and cholestatic serum were used as controls.
Electron microscopy The culture plates were washed with phosphate-buffered saline and fixed in cold 25 g/L glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 48 h. After fixation, the cells were curetted and centrifuged to form aggregates. After post-fixing in 10 g/L osmium tetroxide in 0.1 mol/L sodium cacodylate (pH 7.4), the cells were dehydrated in a graded series of alcohol and embedded in low viscosity epoxy resin. Ultra-thin sections were stained with uranyl acetate and lead citrate and viewed under an electron microscope.
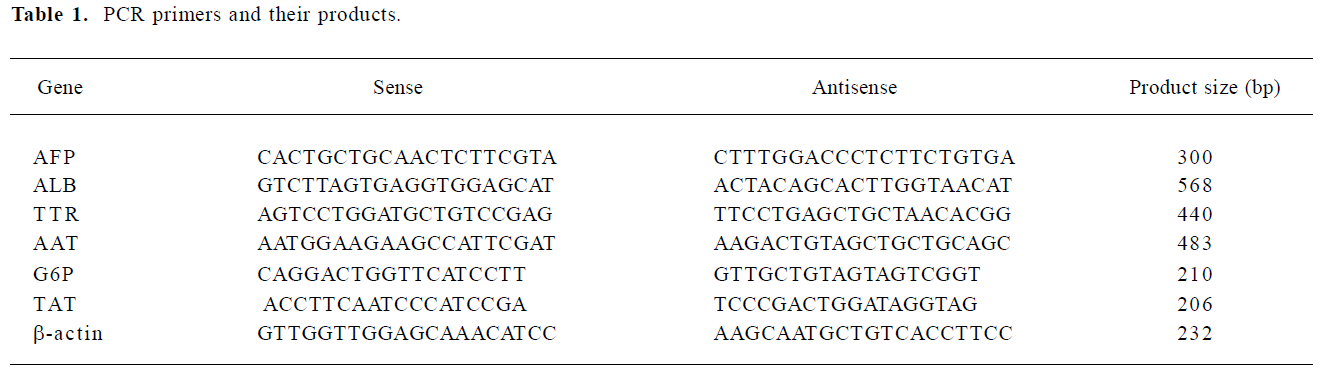
RT-PCR On d 0, 3, 6, 9, 12, 15, 18, and 21 of cell differen-tiation, the cells were collected for an analysis of the mRNA expression of the following liver-specific genes: α-fetoprotein (AFP), albumin (ALB), transthyretin (TTR), α1-antitrypsin (AAT), glucose-6-phosphate (G6P), and tyrosine aminotransferase (TAT). Total RNA was extracted from 1×106 differentiated cells by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was prepared from 2 µg of total RNA by using a RevertAid first-strand cDNA synthesis kit (Fermentas, Hanover, MD, USA). Samples of cDNA corresponding to the input RNA were amplified in PCR reaction buffers containing primers and LA Taq DNA polymerase (Takara, Kyoto, Japan). The primer sequences and the expected sizes of the RT-PCR products are shown in Table 1. The PCR reaction was performed with the following thermal profiles: denaturation at 94 °C for 5 min, followed by 30 cycles of 30 s at 94 °C, 30 s at 55 °C, and 45 s at 72 °C. Then the program was finished by a 10 min extension at 72 °C. The amplified products were subjected to electrophoresis in 20 g/L agarose gels and stained with ethidium bromide. All the procedures were performed according to the manufacturers’ instructions.
Full table
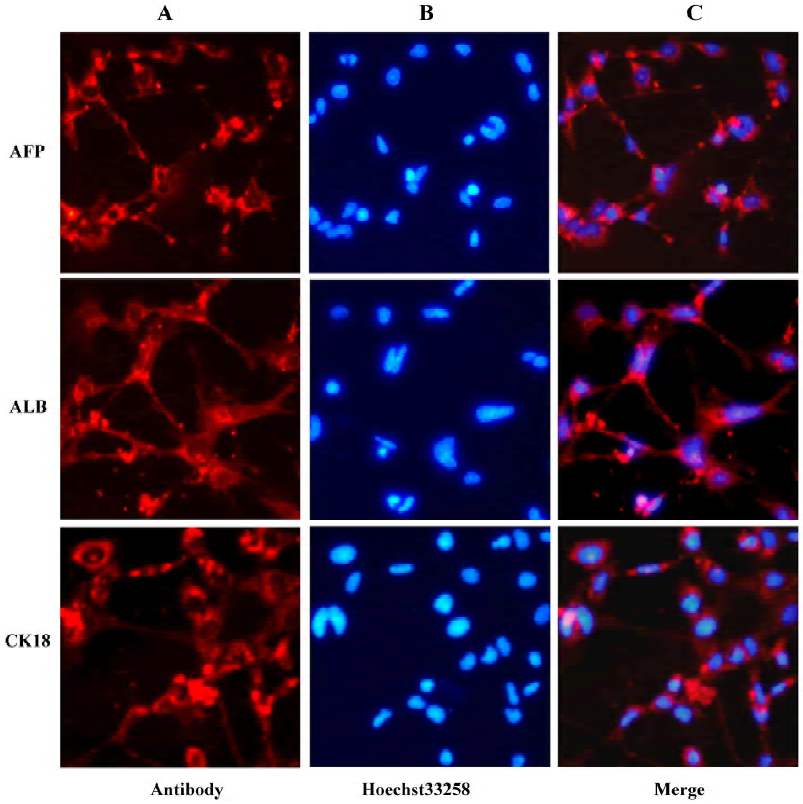
Immunocytochemistry On d 18 of cell differentiation, immunocytochemistry for AFP, ALB, and cytokeratin 18 (CK18) was performed using the methods described previously[22,23]. The primary antibodies were obtained and diluted as follows: goat monoclonal antimouse AFP antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), sheep monoclonal antimouse ALB antibody (1:50; Biodesign, Saco, ME, USA), goat monoclonal antimouse CK18 antibody (1:200; Santa Cruz Biotechnology, USA), and relative Cy3-conjugated secondary antibodies (Sigma, USA). Nuclear DNA was dyed with Hoechst 33258 (Sigma, USA). Hepatocytes from mouse livers were used as controls.
Glycogen detection (Periodic acid–Schiff reaction) The cells cultured on plates were dried in air and fixed with 95% alcohol for 10 min. After rinsing 3 times with distilled water, the cells were oxidized in 1% periodic acid for 10 min, rinsed in distilled water again, then exposed to the Schiff reagent for 10 min at 37 °C. A third water wash to remove the reagent was followed by an inspection of the cells with a light microscope. Spontaneously-differentiated cells were used as controls.
Determination of the hepatic differentiation ratio On d 18 of cell differentiation, immunocytochemistry for ALB was performed as described above. One hundred cells per well and ALB-positive cells within the sample were enumerated; the percentage of ALB-positive cells was determined. The values from each well were averaged to obtain a mean±SD. After staining with periodic acid-Schiff (PAS), the percentage of PAS-positive cells was also evaluated using the same method. The cells cultured with sodium butyrate, but without cholestatic serum, and spontaneously-differentiated cells were used as controls.
Results
Preparation of the conditional selective medium Ten days after the ligation of the common bile duct, the level of serum total bilirubin (STB) in the cholestatic rats was 104±46.2 µmol/L, obviously higher than that in the parallel normal rats (data not shown). The STB concentrations of the conditional selective medium containing 20, 50, and 100 mL/L of cholestatic serum were 2.9, 5.6, and 10.2 µmol/L, respectively.
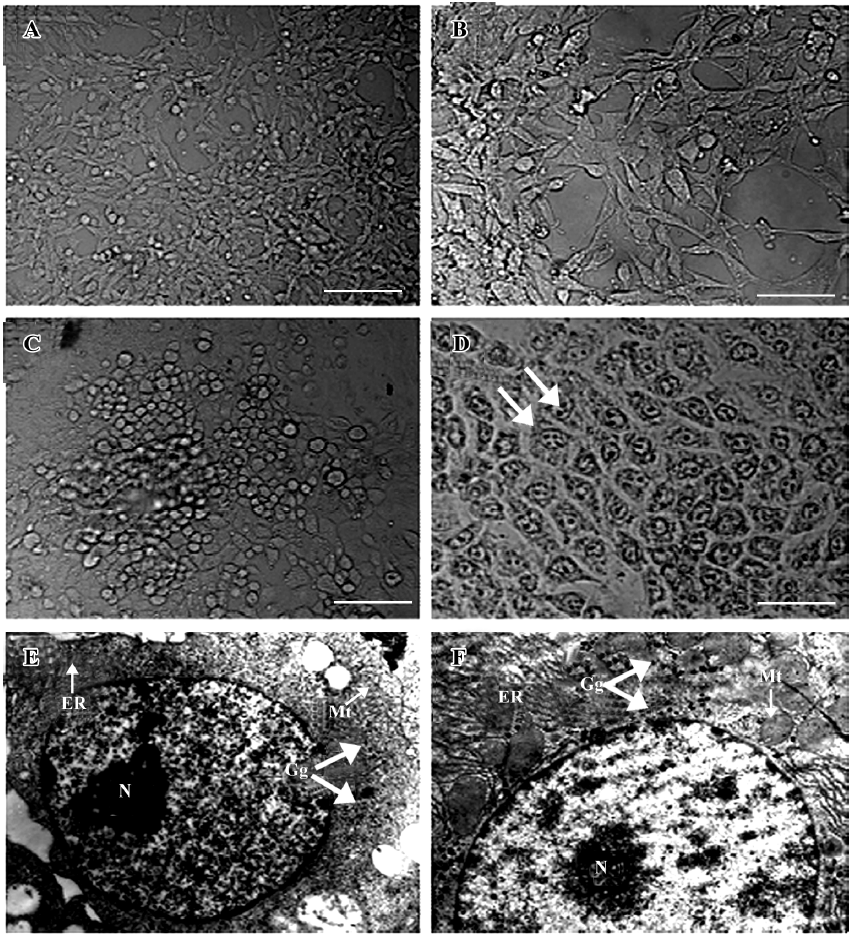
ES cell differentiation into hepatocyte-like cells The morphological changes of the ES cells during the process of cell differentiation were observed using light microscopy. The mouse ES cells proliferated vigorously and EB were formed in the suspension culture 4 d after the removal of LIF. In the absence of sodium butyrate, the control ES cells spontaneously differentiated into multiple lineages (Figure 1A,1B). One day after incubation in culture medium containing sodium butyrate, cell differentiation occurred. By 3 d of culture, cell death was observed in a significant number of cells, while the remaining cells assumed homogenous morphological changes. Most of these cells were epithelioid and fibroblast-like cells. In the presence of conditional selective medium, however, cell proliferation was considerable and numerous epithelial cells resembling hepatocytes were observed (Figure 1C, 1D). The cells were polygonal in shape with large, round, and center-situated nuclei; bi-nuclei were also present. Furthermore, electron micrographs revealed that the differentiated cells were rich in endoplasmic reticulum, ribosomes, ellipsoid mitochondria, and glycogen (Figure 1E), which are typical ultrastructural features of hepatocytes (Figure 1F). Furthermore, we also found that conditional selective medium with 50 mL/L cholestatic serum was optimal for hepatocyte-like cells to survive and proliferate, while 100 mL/L cholestatic serum led to significant cell death, and 20 mL/L cholestatic serum reduced the number of hepatocyte-like cells as compared with 50 mL/L cholestatic serum.
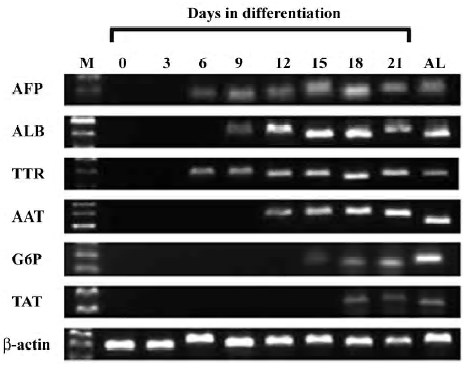
Detection of hepatic markers The results of the RT-PCR analysis showed that undifferentiated ES cells did not express any hepatic markers; however, in the presence of sodium butyrate and conditional selective medium, hepatic differentiation markers were detected. AFP and ALB were expressed within d 6 and d 9. Other endodermal and hepatic markers, TTR and AAT, were also expressed within d 6 and d 12. Late fetal hepatic markers, G6P and TAT, were not expressed until d 15 and d 18, respectively. Moreover, the protein expressions of AFP, ALB, and CK18 were observed in the cells treated with sodium butyrate and conditional selective medium, but not in the ES cells. The experiments were repeated 3 times and the representative data are shown in Figure 2 and 3.
Functional test of differentiated cells Glycogen storage is an important metabolic function of hepatocytes. In this study, the capability of glycogen storage in the differentiated cells was analyzed by the PAS reaction. Glycogen storage was manifested as the accumulation of magenta staining in the cytoplasm of hepatocyte-like differentiated cells. Very few PAS-positive cells were detected amongst the spontaneously-differentiated cells (Figure 4A).
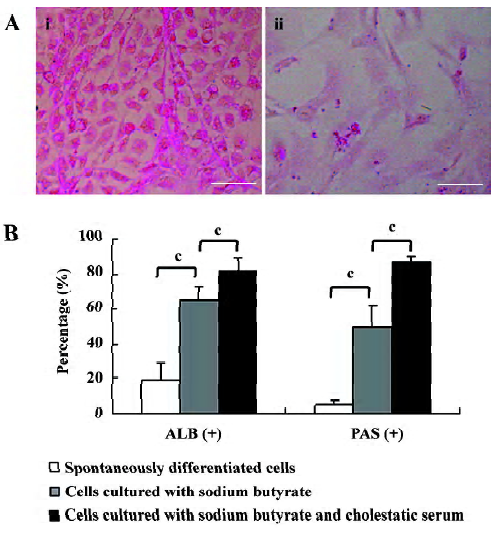
Determination of hepatic differentiation ratio The ratio of hepatic differentiation has rarely been reported in previous studies involving ES cells[24]. In the current study, the hepatic differentiation ratio was determined by evaluating the percentages of ALB- and PAS-positive cells in differentiated cells. On d 18 of cell differentiation in the culture system containing sodium butyrate and cholestatic serum, ALB and PAS staining was observed in 82% and 87% of the cells, respectively. In contrast, in the same culture system without cholestatic serum, ALB and PAS were expressed in 65% and 50% of the cultured cells, respectively. On average, in the spontaneously-differentiated cells, 19% of the cells expressed ALB and 5% of the cells were PAS positive (Figure 4B).
Discussion
In this study, we present a novel and highly efficient method for generating functional hepatocytes from ES cells. Although ES cells can differentiate into hepatocyte-like cells either spontaneously or induced by exogenous factors in vitro[1,19–22], the resulting cells still contain multiple heterogeneous lineages which are not suitable for therapeutic transplantation[25,26]. To avoid the risk of teratoma formation after cell transplantation, recent reports have highlighted the importance of acquiring functional ES-derived hepatocytes with uniform phenotype[24,27]. We found that a culture system containing cholestatic serum was very helpful to selectively enrich and isolate functional hepatocytes from the mixed differentiated ES cells.
The first step in our protocol involved culturing the EB with sodium butyrate, an inhibitor of histone deacetylase that can induce ES cells to differentiate into hepatocytes[13,28,29]. In comparison to the method described previously[28], the present protocol did not contain DMSO. We demonstrated that sodium butyrate alone could efficiently initiate hepatic differentiation from ES cells. The expressions of AFP and ALB mRNA were detected within d 6 and d 9 as compared with d 9 and d 12 in spontaneously-differentiated ES cells. Upon consecutive induction, late fetal hepatic markers, G6P and TAT, were expressed within d 15 and d 18. Moreover, 65% and 50% of the differentiated cells treated with sodium butyrate were ALB and PAS positive on d 18 of differentiation, while only 19% and 5% were ALB and PAS positive in spontaneously-differentiated ES cells. Therefore, our data confirm that hepatocyte-like cells from spontaneously-differentiated ES cells may arrest in an immature stage, while sodium butyrate not only promotes hepatic differentiation from ES cells, but also induces maturation of ES-derived hepatocyte-like cells.
To enrich the hepatocyte-like cells and eliminate the heterogeneous populations, a conditional selective medium containing cholestatic serum was used 7 d after the induction by sodium butyrate. It has been reported that cholestasis after bile duct ligation might induce marked hepatic stem cell proliferation[16], and sera from patients with liver failure could stimulate mouse bone marrow cells to transdifferentiate into hepatocytes[30]. In the present study, we found that cholestatic serum could selectively enrich and isolate ES-derived hepatocytes efficiently. After being treated with cholestatic serum for several days, the differentiated ES cells exhibited very uniform morphology resembling mouse hepatocytes, with many hepatic characteristics in the gene expression profile, phenotypic markers, and functional features. In comparison to the treatment with sodium butyrate alone (without cholestatic serum), the ALB- and PAS-positive cells increased to 82% and 87%, respectively, and no karyotypic and phenotypic changes were observed in the ES-derived hepatocyte-like cells in the presence of cholestatic serum. This indicated that cholestatic serum did not have a negative effect on the procedure of hepatic differentiation mediated by sodium butyrate, but the approximate percentage between PAS- and ALB-positive cells suggested that cholestatic serum could further promote the maturity of ES-derived hepatocyte-like cells.
The efficacy of the conditional selective medium used in this study may be ascribed to cholestatic serum containing the metabolic products which accumulated following common bile duct ligation and hepatic insufficiency (i.e. bilirubin, bile acid, endotoxin, and ammonia). The current study suggests that ES-derived hepatocyte-like cells are capable of accumulating glycogen and express enzymes concerned in metabolism such as G6P and TAT, and it has previously been reported that ES-derived hepatocyte-like cells metabolize ammonia and synthesize urea[13,22]. Thus, we assumed that the functional hepatocyte-like cells metabolized toxic products contained in cholestatic serum, survived, and selectively proliferated in response to the signals characteristic of cholestatic serum, while the non-hepatic cells could not adapt to such a pathological environment and resulted in apoptosis. Therefore, the conditional selective medium based on cholestatic serum selectively enriched hepatocyte-like cells from mixed differentiated ES cells in 2 ways: (1) providing selective proliferative signals for hepatocyte-like cells; and (2) eliminating non-hepatic populations.
In summary, we have demonstrated a novel method to improve the hepatic differentiation ratio with a conditional selective medium containing cholestatic serum. The present results make an important contribution by providing a novel source of donor hepatocytes for therapeutic transplantation.
References
- Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett 2001;497:15-9.
- Ziegler BL, Valtieri M, Porada GA, De Maria R, Muller R, Masella B, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science 1999;285:1553-8.
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyo-cytes. J Clin Invest 2001;108:407-14.
- Rolletschek A, Blyszczuk P, Wobus AM. Embryonic stem cell-derived cardiac, neuronal and pancreatic cells as model systems to study toxicological effects. Toxicol Lett 2004;149:361-9.
- Kim DW. Efficient induction of dopaminergic neurons from embryonic stem cells for application to Parkinson’s disease. Yonsei Med J 2004;45:1203.
- Meyers C. Cruel choices: autonomy and critical care decision-making. Bioethics 2004;18:104-19.
- Nakayama T, Momoki-Soga T, Yamaguchi K, Inoue N. Efficient production of neural stem cells and neurons from embryonic stem cells. Neuroreport 2004;15:487-91.
- Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development 2000;127:2447-59.
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development 2004;131:1651-62.
- Sone M, Itoh H, Yamashita J, Yurugi-Kobayashi T, Suzuki Y, Kondo Y, et al. Different differentiation kinetics of vascular progenitor cells in primate and mouse embryonic stem cells. Circulation 2003;107:2085-8.
- Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Binetruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J 2002;361:621-7.
- Kramer J, Hegert C, Guan K, Wobus AM, Muller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev 2000;92:193-205.
- Sharma NS, Shikhanovich R, Schloss R, Yarmush ML. Sodium butyrate-treated embryonic stem cells yield hepatocyte-like cells expressing a glycolytic phenotype. Biotechnol Bioeng 2006;94:1053-63.
- Hoffman AL, Rosen HR, Ljubimova JU, Sher L, Podesta LG, Demetriou AA, et al. Hepatic regeneration: current concepts and clinical implications. Semin Liver Dis 1994;14:190-210.
- Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 2001;33:738-50.
- Olynyk JK, Yeoh GC, Ramm GA, Clarke SL, Hall PM, Britton RS, et al. Gadolinium chloride suppresses hepatic oval cell proliferation in rats with biliary obstruction. Am J Pathol 1998;152:347-52.
- Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl 2001;7:835-44.
- Cai YF, Zhen ZJ, Min J, Fang TL, Chu ZH, Chen JS. Selection, proliferation and differentiation of bone marrow-derived liver stem cells with a culture system containing cholestatic serum in vitro. World J Gastroenterol 2004;10:3308-12.
- Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transpl 2003;9:1094-9.
- Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology 2003;37:983-93.
- Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM. Hepatic differentiation of murine embryonic stem cells. Exp Cell Res 2002;272:15-22.
- Ishii T, Yasuchika K, Fujii H, Hoppo T, Baba S, Naito M, et al. In vitro differentiation and maturation of mouse embryonic stem cells into hepatocytes. Exp Cell Res 2005;309:68-77.
- Hoppo T, Fujii H, Hirose T, Yasuchika K, Azuma H, Baba S, et al. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology 2004;39:1362-70.
- Hu AB, Cai JY, Zheng QC, He XQ, Shan Y, Pan YL, et al. High-ratio differentiation of embryonic stem cells into hepatocytes in vitro. Liver Int 2004;24:237-45.
- Kumashiro Y, Teramoto K, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Isolation of hepatocyte-like cells from mouse embryoid body cells. Transplant Proc 2005;37:299-300.
- Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, et al. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc 2005;37:285-6.
- Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol 2006;24:1412-9.
- Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant 2003;12:1-11.
- Zhou QJ, Xiang LX, Shao JZ, Hu RZ, Lu YL, Yao H, et al. In vitro differentiation of hepatic progenitor cells from mouse embryonic stem cells induced by sodium butyrate. J Cell Biochem 2007;100:29-42.
- Yamazaki S, Miki K, Hasegawa K, Sata M, Takayama T, Makuuchi M. Sera from liver failure patients and a demethylating agent stimulate transdifferentiation of murine bone marrow cells into hepatocytes in coculture with nonparenchymal liver cells. J Hepatol 2003;39:17-23.
