Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide1
Introduction
Normal penile erection depends on vascular smooth muscle relaxation in erectile tissue and penile arteries, with the main mediator of relaxation being nitric oxide (NO)[1]. Evidence suggests that oxidative stress mediated through the superoxide radical (superoxide) and other reactive oxygen species (ROS) may be central to impaired cavernosal function in erectile dysfunction (ED)[2]. The inactivation of NO by superoxide results in impaired penile NO transmission and smooth muscle relaxation. Given our current understanding of ED pathophysiology, antioxidants may be beneficial in alleviating penile ED in the short and long term.
Berberine (Ber), an isoquinoline alkaloid, is a well-known component of the Chinese herb Huanglian (Coptis chinensis Franch) of the family Berberidaceae. The compound exhibits pharmacological activities of antioxidant action[3,4]. In our previous study, Ber was found to induce NO-mediated relaxation of rabbit corpus cavernosum[5]. Therefore, we aimed to observe the effects of Ber on rabbit corpus cavenosum smooth muscle cells (CCSMC) injured by hydrogen peroxide (H2O2) and to understand the role of Ber in the prevention of ED.
Materials and methods
Animals Male New Zealand rabbits (22–26 weeks old, 3–4 kg body weight; supplied by the Animal Services Center, Yunyang Medical College, Shiyan, China) were used. The rabbits had free access to a standard diet (Altromin pellets) and tap water until use. Fasting was achieved by depriving food but not drinking water 16 h before surgery.
Isolation and culture of CCSMC The CCSMC were isolated from male New Zealand rabbits as described previously[6]. Briefly, the penises were excised at the point of adhesion to the lower pubic bone. The organs were cleared from fat and connective tissue. The facia penis, glans penis, corpus spongiosum, and tunica albuginea were carefully removed. The corpus cavernosum was washed in sterile phosphate-buffered saline (PBS), cut into small strips, and placed for 12–18 h at 37 °C in a tube with 5 mL PBS-buffered minimum essential medium (MEM) containing 0.1% collagenase (type I). After incubation, the pieces were rinsed with PBS-buffered MEM and then mechanically dispersed with a pipette (tip inside diameter 0.5 mm) in the medium. Dissociated cells were collected by centrifugation at 1000 r/min for 5 min and resuspended in 5 mL complete MEM (supplemented with 20% fetal bovine serum, 2 mmol/L L-glutamine, 100 units/mL penicillin G sodium, and 100 µg/mL streptomycin sulfate). The cells were seeded in a 75 cm2 culture flask and cultured undisturbed for 2–3 d in a humidified 5% CO2–95% air incubator at 37 °C. After inoculation for 3 d, the anchorage-dependent cells radially spread, and part of the area was confluent. If the color of the medium became yellow (ie, pH <7), the medium was changed immediately to discard the suspension cells and broken fiber and cells. Afterward, the medium was changed every 2–3 d according to the condition.
The cells were subcultured after attaining >90% conflu-ency (at approximately 10–14 d). The previous medium was decanted from the culture flask, PBS was added to wash the cells, and the cells were detached with 0.25% trypsin for 30–60 s. When the most of cells became round and the intracellular space became broader as seen on inverted microscopy, complete Dulbecco’s modified Eagle’s medium (DMEM) was added to stop the reaction of trypsin. Then the cells on the wall of the culture flask were mechanically dispersed with a pipette and subcultured at a ratio of 1:2 or 1:3 in the culture flask. The cells from the second to fourth passage were used.
Immunohistochemical staining for α-smooth muscle actin The laboratory procedure for SP immunostaining involved a streptomycin avidin-peroxidase immunochemistry kit (Zhongshan Co, Beijing, China). Briefly, the anchorage CCSMC were digested with 0.25% trypsin for 3–5 min and suspended. The suspended cells were placed in 24-well culture plates, some with coverslips, in complete growth medium and cultured at 37 °C. After 2–3 d, the cells were washed twice with PBS, fixed with 4% formaldehyde-PBS for 10 min, and washed twice with PBS. The cells were permeated with 0.5% Triton-X 100 for 10 min and washed twice with PBS. After being blocked with 5% normal goat serum for 30 min, the cells were incubated with a monoclonal antibody against α-smooth muscle actin (1:100, Chemicon International, Temecula, CA, USA) at 4 °C over-night. After being washed 3 times with 0.01 mmol/L PBS, the cells were incubated with biotin-labeled goat anti-mouse immunoglobulin G (IgG, 1:400) at 37 °C for 30 min. After 3 washings with 0.01 mmol/L PBS, the cells were incubated with horseradish peroxidase-labeled streptomycin avidin (1:400) at 37 °C for 20–30 min, and finally diaminobenzidine (DAB) was added for staining. To ensure the reliability and specificity of the results of the immunohistochemical staining, goat serum and PBS were used to replace the first antibody in our control test.
Effect of Ber on cultured CCSMC injured by H2O2
Cell viability assays In this experiment, the groups of cells included the control group (cultured in medium only), model group (cultured in 1 mmol/L H2O2), and test groups (cultured in different concentrations of Ber and 1 mmol/L H2O2).
A methyl thiazolyl tetrazolium (MTT) assay (Duchefa, The Netherlands) to measure cell viability was performed as described previously[7]. The CCSMC were digested with 0.25% trypsin and seeded at 2×104 cells/well in 200 µL in 96-well culture plates. The cells were first cultured for 2–3 d in MEM medium supplemented with 10% fetal bovine serum under normal conditions, then 24 h in medium containing 5% fetal bovine serum. Subsequently, the cells were incubated with Ber (0.1–1000 µmol/L) for 24 h, followed by 4 h stimulation with 1 mmol/L H2O2, and then incubated for an additional 4 h at 37 °C with 20 µL 0.5 mg/mL MTT. The medium was removed and 150 µL/well DMSO was added to resolve the formazan salts. The plates were shaken for 10 min to allow formazan dissolution and then absorbance was measured on a microplate reader at 490 nm. The cell survival rate was calculated: (absorbance of treated group–absorbance of blanks)/(absorbance of control–absorbance of blanks)×100 as a percentage.
Measurement of NO products NO undergoes a series of reactions with several molecules present in biological fluid, leading to the accumulation of the final products, nitrate, and nitrite (NO2– and NO3–). Thus, the index of total production is the sum of both NO2– and NO3– accumulated in the tissue samples. The CCSMC were seeded at 2×104 cells/well in 96-well culture plates and treated as described above. The cell supernatant was collected and the NO products were determined according to the manufacturer’s (Nanjing Jian-cheng Bioengineering Institute, Nanjing, China) recommendation on a microplate reader at 550 nm.
Lactate dehydrogenase release Cell injury induced by H2O2 was quantitatively assessed by the measurement of lactate dehydrogenase (LDH) released from damaged or destroyed treated cells, and an aliquot of bathing media was combined with NADH and pyruvate solutions. LDH activity proportional to the rate of pyruvate loss was assayed according to the manufacturer’s (Nanjing Jiancheng Bioengineering Institute, China) recommendation on a microplate at 440 nm.
Determination of superoxide dismutase activity The superoxide dismutase (SOD) activity of the treated cell supernatant was determined on a microplate at 550 nm. One unit of SOD was defined as the amount required to inhibit the rate of reduction of cytochrome c by 50%.
Assessment of malondialdehyde content Malon-dialdehyde (MDA), an end product of lipid peroxidation, reacts with thiobarbituric acid to form a colored substance. The CCSMC were seeded at 2×104 cells/well in 96-well culture plates and treated. Then the medium was discarded and the cells were washed twice with PBS. Subsequently, a solution of 1 mL 0.1 mmol/L PBS-0.05 mmol/L EDTA (pH 8.0) and 50 µL 1% Triton-X 100 was added into the well to lyse the cells. The MDA content of the cells was determined on a microplate reader at 532 nm. The protein concentrations were determined by Coomassie brilliant blue method.
Drugs and chemicals Ber (chemical purification>98%) was purchased from Sigma (St Louis, MO, USA). DMEM (high glucose) medium and collagenase type I were from Gibco (USA). Trypsin (1:250) and L-glutamine were from Amresco (Solon, Ohio, USA). MTT was from Duchefa (The Netherlands). The detection kits for SOD, MDA, NO, LDH, and Coomassie brilliant blue G250 were from Nanjing Jiancheng Bioengineering Institute (China). Fetal bovine serum was from Hangzhou Sijiqing Biological Engineering Materials (Hangzhou, China).
Statistical analysis Data are expressed as mean±SEM. Differences between groups were assessed by the Student’s t-test. P<0.05 was considered significant.
Results
Morphology and verification of CCSMC After culture for approximately 10–14 d, the CCSMC grew radially and a group of cells grew in parallel along their longitudinal axis, which showed obvious orientation (Figure 1). The CCSMC were verified as spindle-shaped cells by anti-α-actin monoclonal antibody immunohistochemical staining (Figure 2).

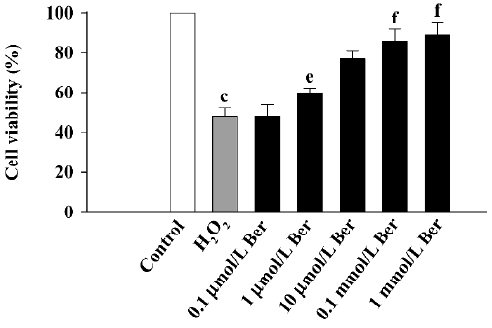
Effects of Ber on CCSMC injured by H2O2 Pretreatment with 1 mmol/L H2O2 decreased the cell viability from 100% for control cells to 48.57%±4.1% (P<0.01), and treatment with Ber (10–1000 µmol/L) inhibited the effect of H2O2 (P<0.05 or P<0.01; Figure 3).
The NO level in the CCSMC was far lower (66.8±16.3 µmol/L) than that in the endothelial cells (569±20.3 µmol/L). Treatment with 1 mmol/L H2O2 decreased the level of NO from 66.8±16.3 to 6.7±2.1 (P<0.01). However, incubation with 1–1000 µmol/L Ber in a concentration dependent manner increased the level of NO (n=8, P<0.01; Figure 4).

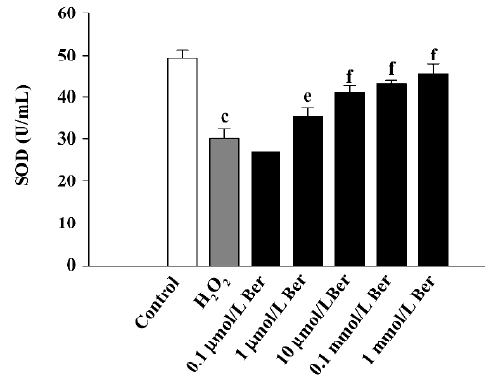
H2O2 treatment increased LDH release, as compared with the control CCSMC, from 497.6±69.5 to 1100.5±56.3 U/L (P<0.01), and treatment with 0.1–1000 µmol/L Ber inhibited this effect (n=8, P<0.05 or P<0.01; Figure 5). SOD activity was decreased with H2O2 treatment from 49.5±1.8 to 30.1±2.6 U/mL (P<0.01), and treatment with 1–1000 µmol/L Ber restored the activity (n=8, P<0.05 or P<0.01; Figure 6).

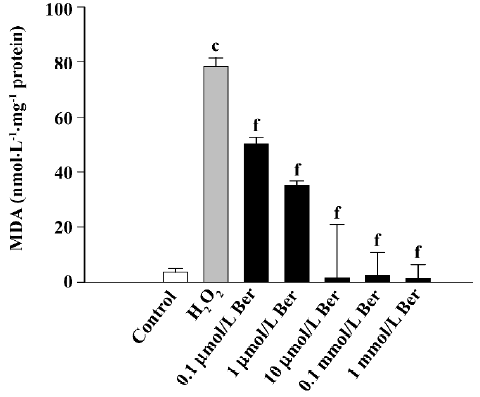
However, 1 mmol/L H2O2 treatment significantly increased the MDA content from 3.7±1.3 to 78.4±2.9 nmol/mg protein (P<0.01), and treatment with 0.1–1000 µmol/L Ber could inhibit this effect (n=8, P<0.01; Figure 7).
Discussion
Oxidative stress, resulting from a disturbance in the balance between the formation of free radicals in the body and their scavenging, is believed to affect the development of endothelial dysfunction and neuropathy within erectile tissues. High levels of ROS in both blood and tissues act as profibrotic factors that stimulate collagen deposition and reduce the ratio of smooth muscle to collagen, thus impairing tissue compliance to relaxation by “hardening” the arterial media[8]. Oxygen-free radicals exert their cytoplasmic effect by peroxidation of membrane phospholipids, which leads to a change in permeability and loss of membrane integrity[9]. Drugs with multiple mechanisms of protective action, including antioxidant properties, may be one way to minimize tissue injury in human diseases.
Our study provides evidence supporting the paradigm of antioxidant therapy for the prevention of ED. We established a H2O2 injured model with cultured rabbit CCSMC and measured cell viability, NO production, and SOD activity; then MDA, a lipid peroxidation product, as an index of oxidative stress; and LDH release, to assess the change in membrane permeability induced by radicals. We found that 1 mmol/L H2O2 significantly increased MDA content and LDH release and decreased cell viability, NO production, and SOD activity. However, treatment with 10–1000 µmol/L Ber inhibited the damage induced by H2O2 by improving cell viability (P<0.05 or P<0.01) and decreasing MDA content (P<0.01) and LDH release (P<0.01).
NO is a mediator of penile erection, and reduced NO levels in the penile corpora is associated with ED[1,9]. With excessive oxidative stress, the reaction of ROS with NO to form peroxynitrite reduces NO concentrations in tissues, which would lead to ED by impairing NO-dependent smooth muscle cell relaxation. Therefore, the effect of Ber on increasing NO production would be beneficial for the prevention of penile ED.
SOD, an antioxidant enzyme, also has an important role in protecting against free radicals by reducing ROS level. We showed that SOD activity was significantly decreased after H2O2 exposure. However, treatment with Ber restored SOD activity.
Our results show that Ber has antioxidant action in CCSMC injured by H2O2 through increasing cell viability, NO production, and SOD activity and decreasing MDA content and LDH release. These effects of Ber can be of benefit in the prevention of ED.
References
- Gonza’lez-Cadavid NF, Ignarro L, Rajfer J. Nitric oxide and cyclic GMP in the penis. Mol Urol 1999;3:51-9.
- Jones RW, Rees RW, Minhas S, Ralph D, Persad RA, Jeremy JY. Oxygen free radicals and the penis. Expert Opin Pharmacother 2002;3:889-97.
- Karimov KhIa. Inoiatova FKh, Salikhodzhaeva USh, Kodiralieva MR. Comparative evaluation of antioxidant properties of neoselen and berberin in chronic heliotrinal hepatitis. Lik Sprava 2002;3–4:123-7.
- Hwang JM, Wang CJ, Chou FP, Tseng TH, Hsieh YS, Lin WL, et al. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch Toxicol 2002;76:664-70.
- Tan Y, Tang Q, Hu B, Xiang J. Modulation action of berberine on NO-cGMP signal pathway in isolated corpus cavernosum smooth muscle. Chin Pharmacol Bull 2005;21:435-40.
- Pilatz A, Schultheiss D, Gabouev AI, Schlote N, Mertsching H, Jonas U, . Isolation of primary endothelial and stromal cell cultures of the corpus cavernosum penis for basic research and tissue engineering. Eur Urol 2005; 47: 710–8; 718–9.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Chou TC, Yen MH, Li CY, Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension 1998;31:643-8.
- Kitamoto S, Egashira K, Kataoka C, Usui M, Koyanagi M, Takemoto M, et al. Chronic inhibition of nitric oxide synthesis in rats increases aortic superoxide anion production via the action of angiotensin II. J Hypertens 2000;18:1795-800.
