Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation1
Introduction
Histone acetylation/deacetylation is a key mechanism for regulating transcription. Acetylation of the ε-amino group of specific lysine residues within the N-terminal tail of core histones results in location chromatin relaxation. In general, histone acetylase activity is correlated with transcription activation, whereas histone deacetylase activity is correlated with transcription repression[1,2]. Significant progress has been made in the use of histone deacetylase inhibitors as antineoplastic drugs. Several reagents have been shown to be histone deacetylase inhibitors (HDACIs), including TSA (Trichostatin A), butyrate, FR90228, and sulindac[3,4]. The mechanism of HDACIs relates to inducing cell cycle arrest and apoptotic responses, and is regulated by changes in histone acetylation and deacetylation. Emerging evidence suggests that a family of histone deacetylases may exist to regulate diverse cellular functions, including chromatin structure, gene expression, cell cycle progression, and oncogenesis[5].
Curcumin, the major component of the spice turmeric and the yellow pigment in curry powder, has been widely used in India and other parts of South-East Asia as a spice and a coloring agent in cooking. Many studies have shown that curcumin (diferuloylmethane) has significantly antiprolifera-tive and apoptotic effects for cancer treatment, including pancreatic carcinoma, liver carcinoma, and leukemia[6,7]. Experimental studies have also revealed that curcumin regulates molecules in the cell signal transduction pathways, including NF-kappaB, Akt, MAPK, p53, AR, Ras, and ER path-ways[8,9]. Research has shown that curcumin is structurally related to sulindac, and the latter is a member of HDACs. Sulindac exerts significant chemopreventive activity, which is related to cell cycle arrest and the histone acetylation/deacetylation state[10]. In a previous study we revealed that curcumin inhibited K562 cell proliferation by Janus kinase-signal transducer and by activating transcription and activator protein-5 signaling pathways[11]. In the present study, we chose the B-NHL cell line as the target. We assumed that curcumin could inhibit carcinoma cell proliferation by regulating Raji cells and we explored the underlying mechanism of curcumin regulating the histone acetylation/deacetylation pathway.
Materials and methods
Drugs and reagents Curcumin was purchased from Sigma Chemical Company (St Louis, MO, USA) and initially dissolved in dimethylsulfoxide (Me2SO), stored at -20 °C, and thawed before use. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) was purchased from Janssen Chimica Company (New Brunswick, NJ), and RPMI-1640 medium, Hoechst 33258, and Me2SO were purchased from Sigma. Anti-HDAC1, anti-HDAC3, anti-HDAC8, and anti-Ac-histone H4 were purchased from Santa Cruz (California, USA). Streptavidin peroxidase (SP) reagent kits were purchased from Zhongshan Company (Beijing, China). Chemiluminescence (ECL) reagent kits were purchased from Pierce Biotechnology, Inc (Rockford, IL). The Raji cell line was obtained from China Center for Typical Culture Collection (Wuhan, China). The following treatments were applied: untreated Raji cells and Raji cells treated with 6.25 µmol/L, 12.5 µmol/L, and 25 µmol/L of curcumin for 24 h. All cell groups were grown in RPMI-1640 culture medium containing 10% fetal calf serum (FCS) and 2 mmol/L L-glutamine at 37 ºC in a 5% CO2 incubator.
MTT assay The antiproliferative effects of curcumin against different cell groups were determined using the MTT dye uptake method. In brief, the cells (40 000 per well) were incubated in triplicate in a 96-well plate. Different concentrations of curcumin were added, and the final concentrations were 6.25, 12.5, 25, 50, and 100 µmol/L. The plates were in the presence or absence of the indicated test samples for 0, 24, 36, 48, 60, and 72 h. The largest Me2SO dissolved concentration group acted as the control group. Thereafter, 20 µL MTT solution (5 g/L in phosphate-buffered saline [PBS]) was added to each well. After 4 h at 37 °C, the supernatant was removed and 150 µL Me2SO was added. When the blue crystal was dissolved, the optical density (OD) was detected in the microplate reader at 570 nm wavelength using a 96-well multiscanner autoreader (Biotech Instruments, New York, USA). The following formula was used: Cell proliferation inhibited (%)=[1–(OD of the experimental samples/OD of the control)]×100%.
Apoptosis assay Curcumin-induced apoptosis was monitored by the extent of nuclear fragmentation. Nuclear fragmentation was visualized by Hoechst 33258 staining of apoptotic nuclei. Apoptotic cells were collected by centrifu-gation, washed with PBS, and fixed in 4% paraformaldehyde for 20 min at room temperature. Subsequently the cells were washed and resuspended in 20 µL PBS before being deposited on poly lysine-coated coverslips and left to adhere to the cover slips for 30 min at room temperature, after which the cover slips were washed twice with PBS. The adhered cells were then incubated with 0.1% Triton X-100 for 5 min at room temperature and rinsed with PBS three times. The coverslips were treated with Hoechst 33258 at 37°C for 30 min, rinsed with PBS, and mounted on slides with glycerol-PBS. The cells were viewed with an Olympus BH-2 fluorescence microscope (Japan).
Immunocytochemistry analysis Curcumin-treated cells were plated onto a glass slide, air dried for 1 h at room tem-perature, and fixed with cold acetone. After brief washing in PBS, the slides were blocked with 5% normal goat serum for 1 h and incubated with HDAC1, HDAC3, HDAC8, and Ac-histone H4 (dilution 1:100, respectively). After being left overnight at 4 ºC the cells were treated with biotinylated link secondary antibody and peroxidase-labeled streptavidin followed by diaminobenzidine (DAB). The cells were viewed with an Olympus microscope and their individual OD values were recorded using an HPIAS 1000 Image Analysis System (High Resolution Pathological Image & Word Analysis System, Bejing, China).
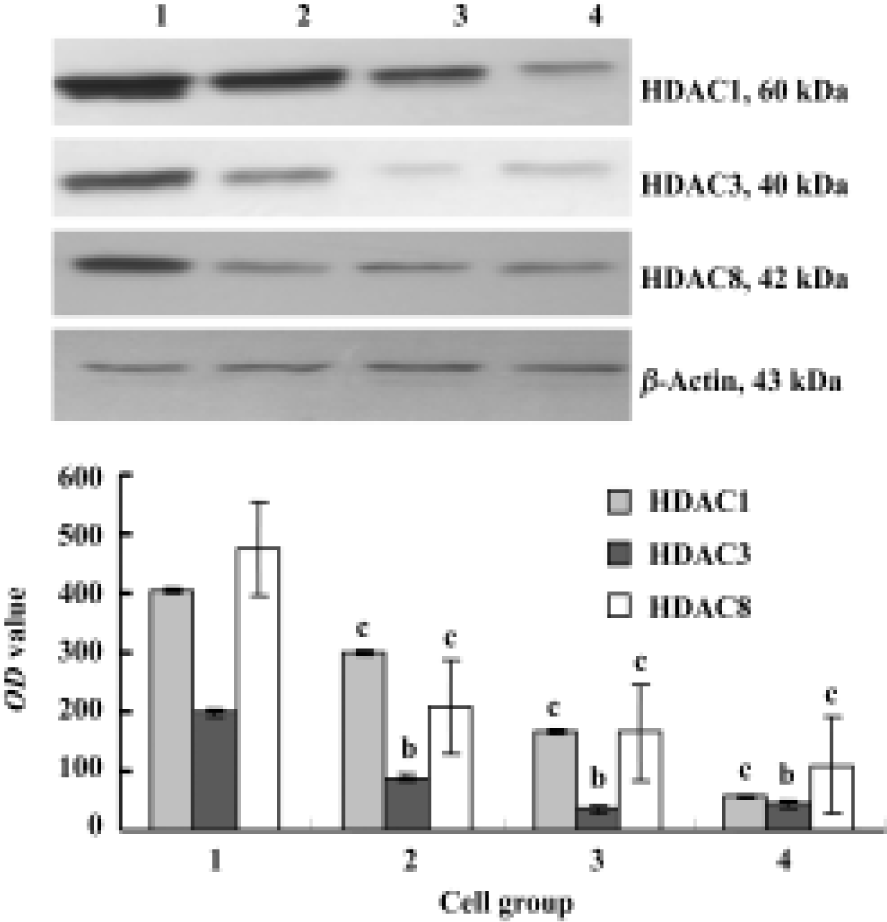
Western blot analysis Lysates were prepared from 1×107 cells by dissolving cell pellets in 100 µL of lysis buffer (Na2PO4 (pH 7.4) 20 mmol/L, NaCl 150 mmol/L, Triton X-100 1%, apro-tinin 1%, phenylmethylsulfonyl fluoride 1 mmol/L, leupeptin 10 g/L, NaF 100 mmol/L, and Na3VO4 2 mmol/L). Lysates were centrifuged at 18 000×g for 15 min and the supernatant was collected. Protein content was determined using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (10 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.2 mol/L DTT) was added to the lysates. Lysates were heated to 100 ºC for 5 min, and 80 µg of protein was loaded into each well of a 10% SDS-PAGE gel. Resolved proteins were electrophoretically transferred to nitrocellulose and blocked with 5% non-fat milk, and the primary antibodies HDAC1, HDAC3, HDAC8, and Ac-histone H4 were added. After overnight incubation at 4 °C the blots were washed, exposed to HRP-conjugated corresponding secondary antibodies for 1 h, and finally detected by ECL. Quantification of the bands was carried out using densitometric analysis software, Quantity One (Bio-Rad), and processed as described previously[12].
Statistical analysis All data were expressed as mean±SD using SPSS 10.0 for windows 98. Using linear t-tests for statistics analysis, P values of less than 0.01 or 0.05 were considered to be statistically significant.
Results
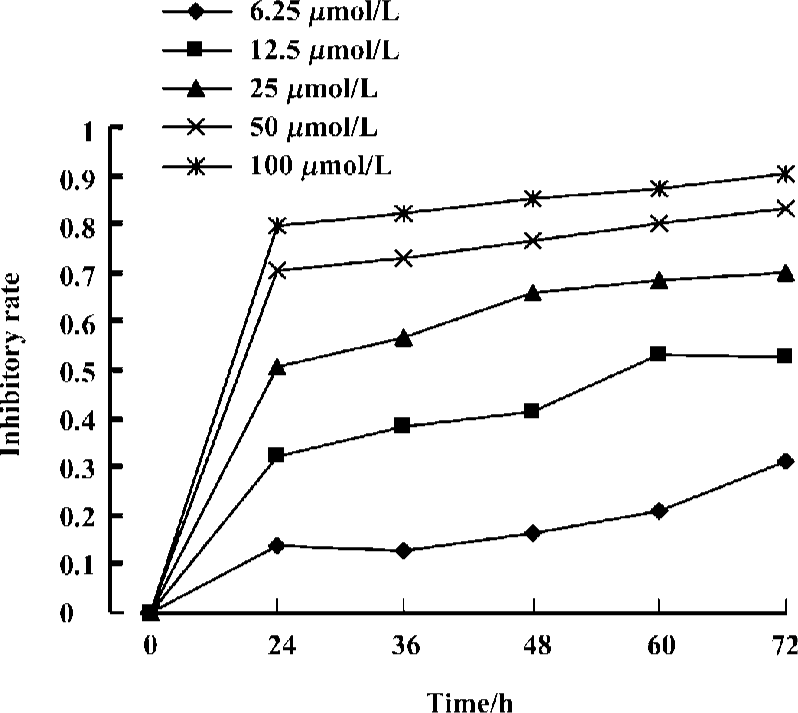
Effects of curcumin on the proliferation of Raji cells by MTT Raji cells treated with different concentrations of curcumin for 0, 24, 36, 48, 60, and 72 h resulted in the inhibition of cell proliferation in a dose- and time-dependent manner. The OD value of curcumin-treated groups decreased significantly compared with the untreated group. Results reveal great differences between curcumin-treated groups and the untreated group (Figure 1). The IC50 of 36 h is 24.1±2.0 µmol/L.
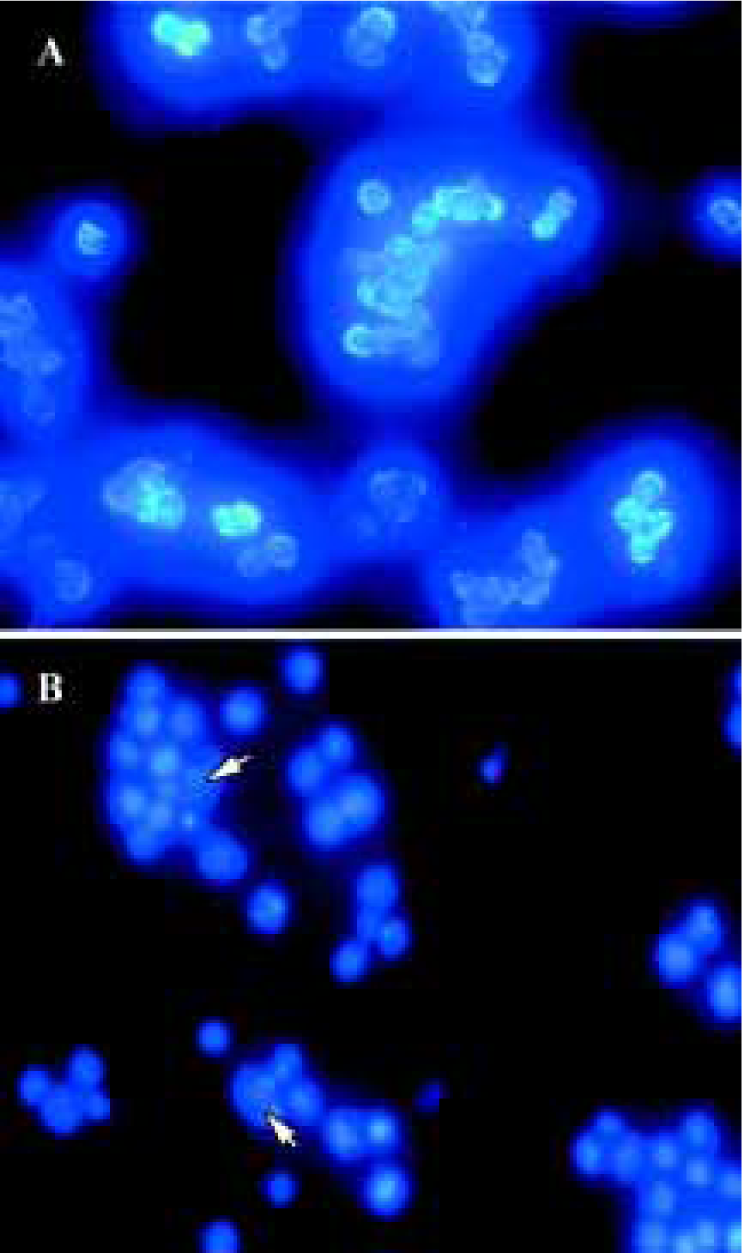
Nuclear damage observed using Hoechst 33258 staining Apoptotic nuclear morphology was assessed using Hoechst 33258 staining. Hoechst 33258 staining of untreated Raji cells and cells treated with 25 µmol/L curcumin for 24 h was conducted. Curcumin permeates the cell and is known to play a role in cancer chemoprevention and tumor growth suppression. Exposure of tumor cells to curcumin in vitro results in the inhibition of cell proliferation and the induction of apoptosis. Consistent with previous reports on other cell lines[9,11], treatment of Raji cells with curcumin (24 h exposure to 25 µmol/L curcumin) induces apoptosis (Figure 2).
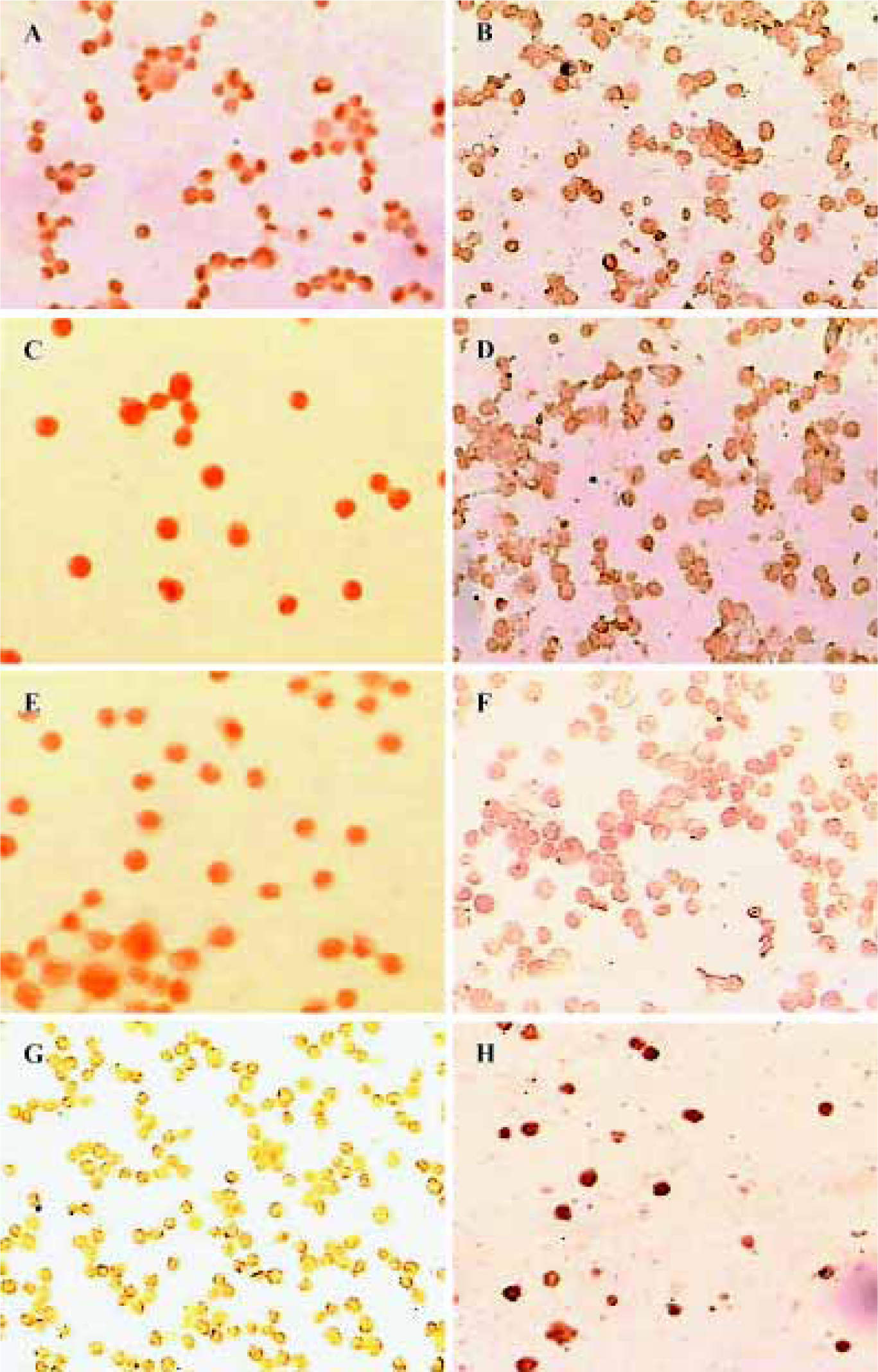
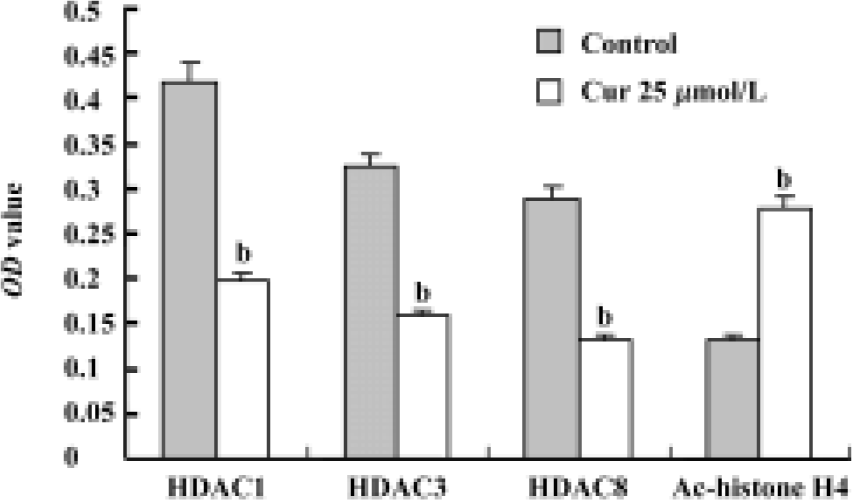
Expression of HDACs and Ac-histone H4 on Raji cells and curcumin-treated cells using immunocytochemistry Our results reveal that the expression of HDAC1, HDAC3, and HDAC8 was significantly higher in Raji cells compared with curcumin-treated cells (25 µmol/L for 24 h)(P<0.05). The expression of Ac-histone H4 was significantly higher in curcumin-treated cells (25 µmol/L for 24 h) than in Raji cells (P<0.05)(Figure 3). Photos were analyzed using a HPIAS 1000 Image Analysis System and OD values were recorded (Figure 4).
Expression of HDAC1, HDAC3, and HDAC8 on Raji cells and curcumin-treated cells using Western blot Our results reveal that curcumin can induce antiproliferation and apoptosis in Raji cells. However, it is unclear how curcumin induces this antiproliferation and apoptosis. Cells treated with 6.25, 12.5, and 25 µmol/L of curcumin for 24 h were lysed and resolved in 10% SDS-PAGE, and Western blot analysis was carried out using anti-HDAC1, anti-HDAC3, and anti-HDAC8. Figure 5 shows considerable changes in HDAC1, HDAC3, and HDAC8 following curcumin treatment. These results indicate that HDAC1, HDAC3, and HDAC8 are related to curcumin-mediated apoptosis. The levels of HDAC1, HDAC3 and HDAC8 protein decreased in a dose-dependent manner (Figure 5).
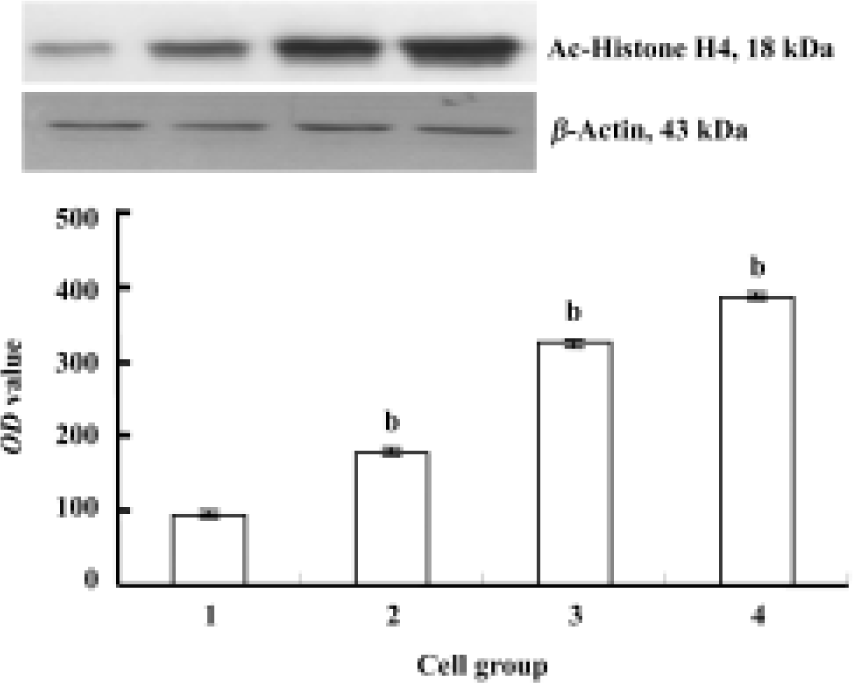
Expression of Ac-histone H4 in Raji cells and curcumin-treated cells The expression of Ac-histone H4 was significantly greater in curcumin-treated Raji cells (6.25, 12.5, and 25 µmol/L, for 24 h) than that in Raji cells (P<0.05, Figure 6).
Discussion
Reversible histone acetylation occurs in the ε-amino group of the specific internal lysine residues located at the highly basic N-terminal domains of core histones. Histone acetyltransferase (HAT) and HDAC control the addition and removal of acetyl groups on proteins and maintain a dynamic balance of steady-state acetylation[13]. A balance between the acetylation and deacetylation states of these proteins forms the basis for the regulation of transcription. Research has shown that HDACs have wide ranges of effects on cell function. These effects include specific gene activa-tion, inhibition of cell proliferation and cell cycle arrest, as well as induction of cell differentiation[14]. Multiple forms of HDACs have been identified in mammalian cells. In humans, at least 11 HDACs have been uncovered. They are classified into three general classes: class I (HDAC1, 2, 3, 8, and 11), class II (HDAC4, 5, 6, 7, 9, and 10) and class III[15]. Class I enzymes are smaller polypeptides of approximately 500 amino acids, whereas class II HDACs are much larger proteins with approximately 100 amino acids. Most class II HDACs shuttle between the cytoplasm and nucleus and regulate myogenesis. HDACs can react with co-repressor (Mad/Max, N-CoR, SMRT) to regulate cell proliferation and change the dynamics of chromatin structure[16]. In general, HDAC1, HDAC3, and HDAC8 are located in the cell nucleus. Many signal transfer pathways (RAS/MAPK, JAK-STAT) and transcriptional factors related to hematopoietic stem cells are regulated by HDACs and HATs.
B-NHL plays an important role in blood system tumors. We chose the Burkkit lymphoma cell line Raji as the research target. Previous studies have revealed that lymphoma is related to the rearrangement of BCL6. Several studies have shown that BCL6 is rearranged in 30%–40% of diffuse large cell lymphoma (DCLC) and 6%–14% of follicular lymphomas (FL). In addition, the chromosomal band 3q27 affects IG gene loci that lead to lymphoma. Abnormal BCL6 can regulate cell-cycle factors (pRB, PLZF) by recruiting HDACs, and can affect the cell cycle. As the result of an abnormal recruiting function, many transcriptional factors can suppress specific genes, which can lead to carcinogenesis. HDACIs can cure cancer by inhibiting HDACs and blocking abnormal recruitment.
Chemoprevention is a rapidly growing field in cancer research that focuses on inhibiting and delaying the onset of carcinogenesis. A large number of natural products have been evaluated as potential chemopreventive agents. Numerous studies have shown that curcumin could suppress the proliferation of many cancer cells. In the present study, we found that curcumin could inhibit the proliferation of Raji cells, and that the effects were time- and dose-dependent. The 36 h IC50 of curcumin was 24.1±2.0 µmol/L. Curcumin can lead to the apoptosis of Raji cells, and can affect cell cycles. The cell cycle was arrested in G0 /G1 and G2/M phases, and the S phase also decreased (data not shown). However, the mechanisms of apoptosis and cell cycle arrest are not clear. In our study, the expression of HDAC1, HDAC3, HDAC8 and Ac-histone H4 on B-NHL cell line Raji and curcumin-treated Raji cells (different concentrations for 24 h) was examined using immunocytochemistry and Western blot analysis. The expression of HDAC1, HDAC3, and HDAC8 proteins on Raji cells decreased compared with the control Raji group, whereas Ac-histone H4 expression increased compared with the control Raji group in a dose-dependent way.
Trichostatin (TSA) was the first discovered HDAC inhibitor (HDACI), followed by sodium butyrate, sulindac, MS-27-275, and FR90228[10]. In vitro and in vivo studies examining HDACIs reveal that HDACIs affect many cell functions, such as cell proliferation, chromosome remodeling, and gene transcription. The mechanism of action relates to inhibiting HDACs, increasing the function of HATs, and increasing histone deacetylation. Hu et al[15] found that TSA decreased the expression of HDAC1, HDAC3, and HDAC8 in SW620 in a dose-dependent way. The IC50 was approximately 0.1–0.3 nmol/L. In addition, TSA can increase the expression of Ac-histone H4 and lead to apoptosis, which is related to the SV40 promotor. Balasubramanyam et al[13] reported that curcumin was a specific inhibitor of p300/CBP HAT activity, but not of PCAF, in vitro and in vivo. Furthermore, curcumin can also inhibit the p300-mediated acetylation of p53 in vivo. Curcumin specifically represses the p300/CBP HAT activity-dependent transcriptional activation from chromatin, but not from a DNA template.
Thus, we believe that curcumin, as a new member of the HDACIs, can inhibit the expression of HDAC1, HDAC3, and HDAC8 in curcumin-treated Raji cells and can increase the expression of Ac-histone H4. In addition, curcumin can inhibit cell proliferation and induce apoptosis. Dysfunction of histone acetyltransferases and histone deacetylases is often associated with the manifestation of several different types of cancer. These enzymes, therefore, are potential new targets for therapy. However, the mechanism by which the abnormal function of HDAC1, HDAC3, and HDAC8 regulates gene transcription remains to be determined. The increase in Ac-histone H4 highlights whose gene will be opened and provides a new field to examine the action mechanism of curcumin.
Acknowledgements
Thanks to the Tumour Biology Laboratory, Center of Gynaecology, Tongji Hospital, Huazhong University of Science and Technology for offering relevant experimental facilities and technical support. We wish to particularly thank Prof Jian-feng ZHOU and Yun-ping LU for their guidance and help with the experiment.
References
- Secrist JP, Zhou X, Richon VM. HDAC inhibitors for the treatment of cancer. Curr Opin Invest Drugs 2003;12:1422-7.
- Hu E, Dul E, Sung CM, Chen Z, Kirkpatrick R, Zhang GF, et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Ther 2003;307:720-8.
- Buggy JJ, Sideris ML, Mak P, Lorimer DD, McIntosh B, Clark JM. Cloning and characterization of a novel human histone deacetylase, HDAC8. Biochem J 2000;350:199-205.
- Murata M, Towatari M, Kosugi H. Apoptotic cytotoxic effects of a histone deacetylase inhibitor, FK228, on malignant lymphoid cells. Jpn J Cancer Res 2000;91:1154-60.
- Graessle S, Loidl P, Brosch G. Histone acetylation: plants and fungi as model systems for the investigation of histone deacetylases. Cell Mol Life Sci 2001;58:704-20.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 2003;23:363-98.
- Leu TH, Maa MC. The molecular mechanisms for the antitumo-rigenic effect of curcumin. Curr Med Chem Anti-Cancer Agents 2002;2:357-70.
- Ranjan D, Chen C, Johnston TD, Jeon H, Nagabhushan M. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappaB activation, and IL-2 signaling. J Surg Res 2004;121:171-7.
- Wu L, Xu J, Wu G, Chen Y. Inhibitory effect of curcumin on proliferation of K562 cells involves down-regulation of p210bcr/abl-initiated Ras signal transduction pathway. Acta Pharmacol Sin 2003;24:1155-60.
- Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res 2000;60:4561-72.
- Chen W, Chen Y, Gu J, He J. Effects of curcumin on proliferation of K562 cells involves STAT5 signal transduction pathway. Chin J Hematol 2004;25:151-3.
- Zhou N, Zhu X, Zhou J, Li M, Zhang X, Huang P, et al. 2-Methoxyestradiol induces cell cycle arrest and apoptosis of nasopharyngeal carcinoma cells. Acta Pharmacol Sin 2004;25:1515-20.
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, et al. Curcumin, a novel p300/CBP specific inhibitor of acetyltransferase, represses the acetylation of histones/nonhistone proteins and HAT dependent chromatin transcription. J Biol Chem 2004;279:51163-71.
- Sullivan SA, Landsman D. Characterization of sequence variability in nucleosome core histone folds. Proteins 2003;52:454-65.
- Hu E, Chen Z, Fredrickson T, Zhu Y, Kirkpatrick R, Zhang GF, et al. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. J Biol Chem 2000;20:15254-64.
- Yamashita Y, Shimada M, Harimoto N, Rikimaru T, Shirabe K, Tanaka S, et al. Histone deacetylase inhibitor trichostatin A induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. Int J Cancer 2003;103:572-6.
