Mycophenolate mofetil as a treatment for refractory idiopathic thrombocytopenic purpura1
Introduction
Idiopathic thrombocytopenic purpura (ITP) is a common autoimmune hemorrhagic disorder, and the treatments are based on individual experience[1]. In the clinic, the first line treatment is steroid therapy, and splenectomy is recommended as an alternative. However, 20% to 30% of ITP patients failed to respond to these two treatments, especially patients with refractory ITP[2]. As the new immunosuppressive agent, mycophenolate mofetil (MMF) is widely used in renal transplantations and its relative safety has already been confirmed[3,4]. In recent times, MMF has been used in the treatment of autoimmune disorders such as nephrotic syndrome[5], Crohn’s disease[6], and autoimmune myasthenia gravis. The immunosuppressive ability of MMF is mainly derived from the inhibition of inosine monophosphate dehydrogenase[7]. It has been reported that MMF can selectively inhibit the proliferation of T and B-lymphocytes, the generation of the antibodies, and the production of the cytotoxic T cells induced by immune stimuli. This also provided a strong basis for MMF as a novel therapeutic agent to treat refractory ITP. Here we reported the long-term therapy with MMF of 20 patients with refractory chronic ITP.
Materials and methods
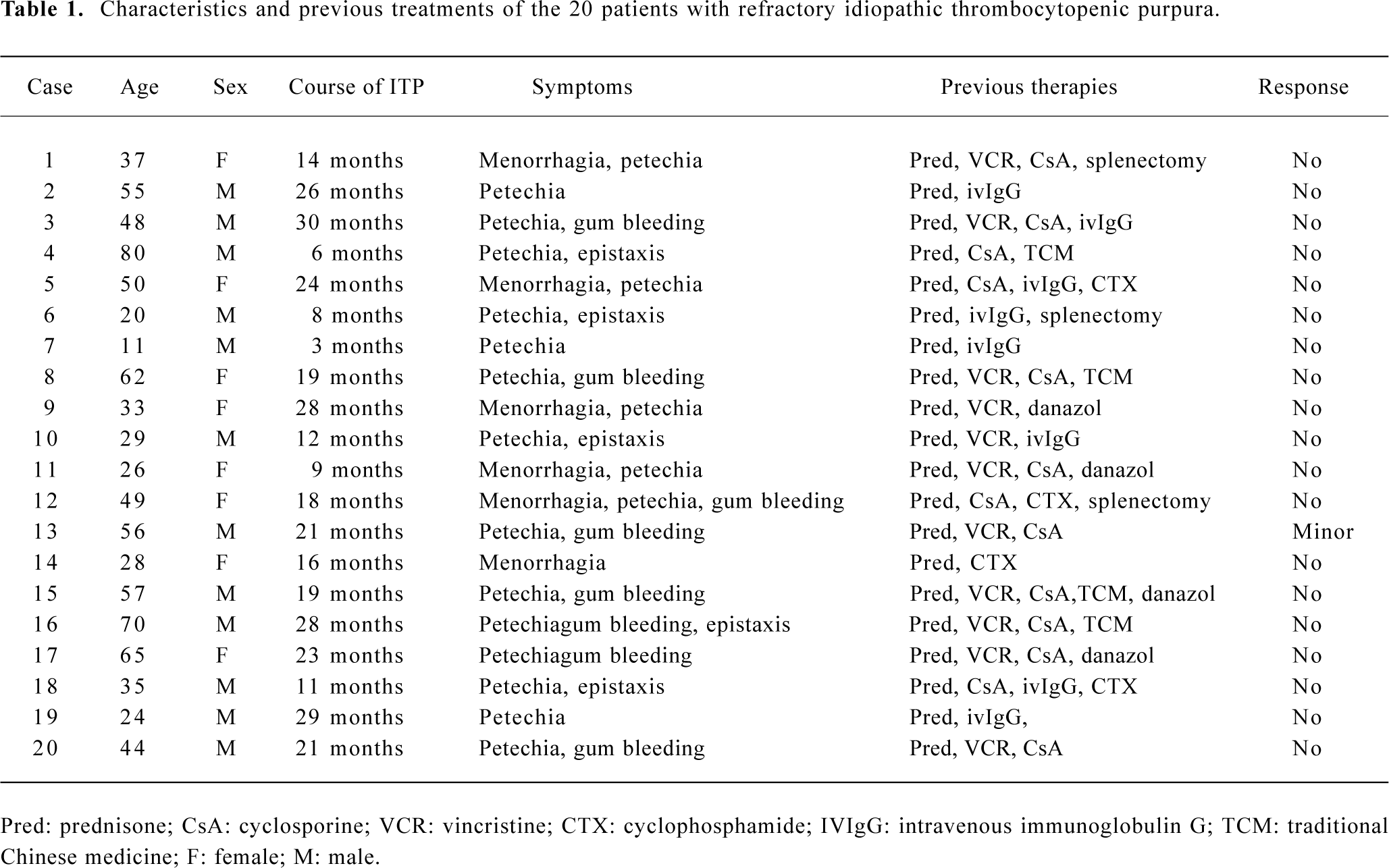
Patients Twenty patients (12 males and 8 females), aged between 11 and 80 years (median 44.3 years) participated in the study; each patient gave their informed consent. The Ethics Committee of the 2nd Hospital of Xi’an Jiaotong University approved the study. Each patient was diagnosed as refractory ITP in accordance with the following criteria: (1) thrombocytopenia (a platelet count<50×109/L) over 6 months, unrelated to any underlying viral infection, collagen vascular diseases, malignancy, or medications; (2) without or with slight splenomegaly, with a normal or increased number of megakaryocytes in bone marrow, and no failure of maturing; (3) the failure of drug treatment and surgery treatment (prednisone, vincristine, danazol, long-term use of traditional Chinese medicine or intravenous injection of high doses of immunoglobulin G and splenectomy) (Table 1).
Full table
Therapeutic regimen MMF (250 mg/capsule, Shanghai Roche Pharmaceuticals Ltd, Shanghai, China) was taken orally at a dosage of 1.5–2.0 g/d for 4 weeks as 1 period of treatment. Patients who had responses to MMF continued taking MMF for another 2–4 periods. The therapy was discontinued in patients who achieved complete response during therapeutic period and in all patients after 16 weeks. Patients were given prednisone (1 mg·kg-1d-1) orally and hemostasia therapy concurrently with MMF. Follow-up was performed on each patient for 2−6 months (median, 3 months). Full blood platelet count was evaluated before and after MMF treatment in every period. Serum immunoglobulin IgG, IgM, and IgA were detected by rate nephelometry with the Array 360 system and associated reagents (Beckman Coulter Inc., Fullerton, CA, USA). Platelet-associated antibodies (PAIgG) were assayed by enzyme-linked immunosorbant assay (ELISA, PeproTech Inc, Rocky Hill, NJ, USA). The normal value of PAIgG is <60 µg/L. Peripheral blood mononuclear cells (PBMNCs) were isolated by a Ficoll gradient (Ficoll-Hypaque, Density=1.077 g/L, Amersham-Pharmacia, Piscata-way, NJ, USA). Fluorescein-conjugated monoclonal antibodies against CD3 (SK7 clone) and CD4 (SK3 clone) and phycoerythrin (PE)-conjugated monoclonal antibodies against CD8 (SK1 clone) were obtained from Becton Dickinson (Mountain View, CA, USA). Appropriate isotype-matched controls were included. PBMNCs were incubated with the monoclonal antibodies on ice for half an hour, then washed in phosphate buffered solution (PBS) three times, and suspended in PBS supplemented with 0.5% bovine serum albumin (BSA). A phenotypic analysis of cell populations was performed on a FACScan flow cytometer by using LYSIS software (Becton Dickinson, Franklin Lakes, NJ, USA). The criteria for response were defined as follows: (1) complete response: thrombocyte count rose above 300×109/L, and blood platelet count rose above 100×109/L. There was no bleeding for at least 3 months and no relapse for 2 years; (2) partial response: blood platelet count was above 50×109/L or 30×109/L higher than that before MMF treatment. There was no bleeding for 2 months; (3) minor response: increase in blood platelet count not exceeding 30×109/L after MMF treatment. Bleeding symptoms were improved for 2 weeks; (4) no response: blood platelet was unchanged and bleeding symptoms were neither improved or worsened.
TUNEL assay Peripheral blood lymphocytes isolated from 20 refractory ITP patients were grown with or without mycophenolic acid 0.1 µmol/L (MPA, Sigma, St Louis, USA) for 3 d. The apoptosis was analyzed by transferase-mediated dUTP-biotin nick-end labeling (TUNEL) using a TUNEL kit (Boster Co, Wuhan, China). TUNEL assay were performed at room temperature unless indicated. Cells were fixed in 4% paraformaldehyde/1×PBS at for 10 min. After pouring off para-formaldehyde cells were incubated with PBS containing glycine 50 mmol/L for 10 min. Cells were permeabilized by 0.5% Triton X-100 in PBS for 10 min. After washing thrice in PBS, cells were equilibrated in equilibration buffer [200 mL of 1×terminal deoxynucleotidyl transferase (TdT) buffer+1 mmol/L cobalt chloride) under a 60 mm×24 mm coverslip for 5 min. Cells were incubated in 1×TdT buffer containing cobalt chloride1 mmol/L, 25 units TdT 100 μL, and 0.25 nmol/L biotin labeled dATP 100 μL at 37 °C for 1 h in humidified chamber (petri dish, lined with filter paper, soaked PBS). The tailing reaction was terminated by 4×standard saline citrate (SSC). After applying 200 μL of 4× SSC with 2 g/L BSA and 1:100 dilution of fluorochrome-avidin under a coverslip, cells were incubated at 37 °C for 1 h in humidified chamber. Then cells were washed in dark in 4×SSC for 5 min, 4×SSC/0.1% Tween 20 with 0.01 g/L DAPI for 5 min, and 4×SSC for 5 min, respectively. The TUNEL-positive cells were analyzed by fluorescence microscopy.
Statistical analysis Statistic analysis was performed by the χ 2 test and the paired t-test. All data are expressed as the mean±SD. P<0.05 was considered to be significant.
Results
Therapeutic effects of MMF Sixteen of the 20 (80%) patients had responses to MMF treatment; 9 (45%) achieved a complete response, 4 (20%) achieved a partial response, and 3 (15%) achieved a minor response. The platelet count increased in 4 patients after 2 weeks of MMF treatment; it was above 50×109/L in 7 patients after 4 weeks of MMF treatment and was above 100×109/L in 10 patients after 6 weeks of MMF treatment. The responses of the 16 patients were sustained overtime after the withdrawal of MMF for 1 month, but less patients had a relapse after treatment discontinuation.
Twelve men had responses to MMF treatment; 8 (66.7%) achieved a complete response, 2 (16.7%) achieved a partial response, and 2 (16.7%) achieved a minor response. From the 8 women, only 1 (12.5%) achieved a complete response, 2 (25%) achieved a partial response, 1 (12.5%) achieved a minor response, and 4 (50%) had no response. These results indicate that the therapeutic effects of MMF are relatively better in male patients than female patients.
Side effects of MMF During the early stages of treatment, 7 patients experienced transient adverse reactions such as abdominal distension, anorexia, and nausea; these symptoms were later treated. No blood infection, bone marrow suppression, hypertension, severe headache, or muscle pain was observed in the 20 patients after MMF treatment. All of the patients completed the scheduled treatment suggesting that these patients had no tolerarance of MMF.
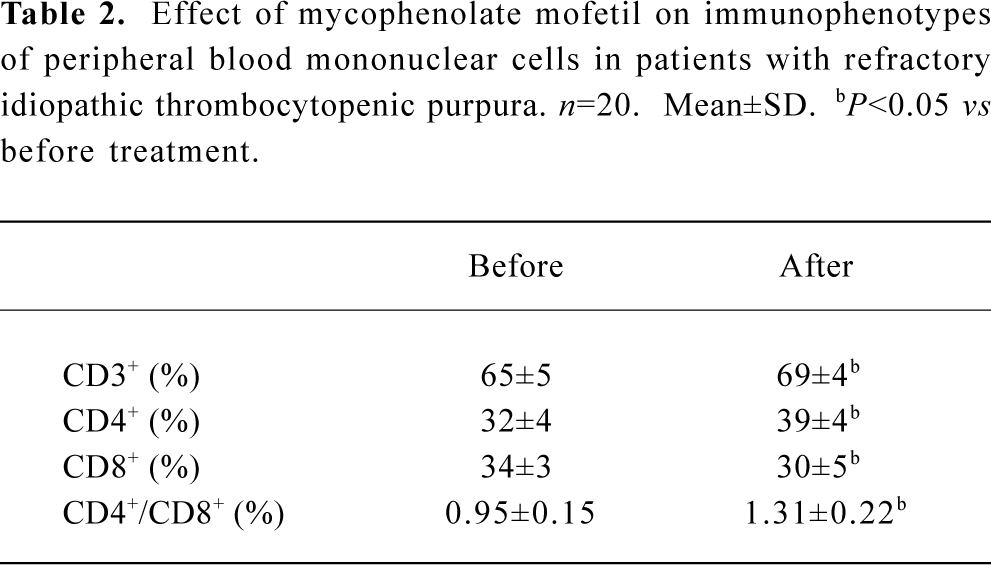
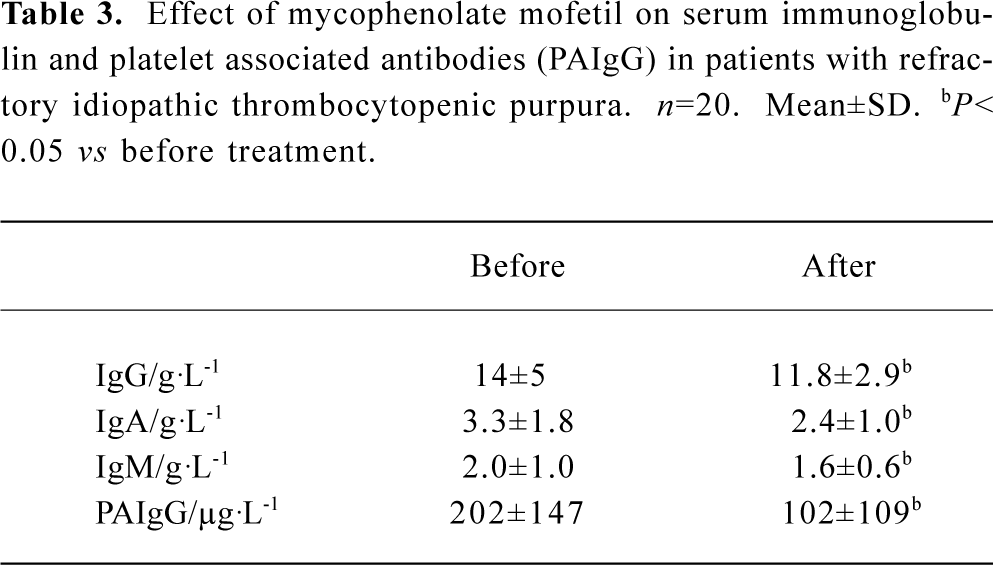
Effect of MMF on immunophenotypics The percentage of CD3+ and CD4+ lymphocytes increased, but the percentage of CD8+ lymphocytes decreased. Thus, the CD4+/CD8+ ratio elevated after MMF treatment (Table 2). The plasma levels of IgG, IgM, IgA, and PAIgG were markedly reduced in 17 patients after more than 2 weeks of MMF treatment (Table 3).
Full table
Full table
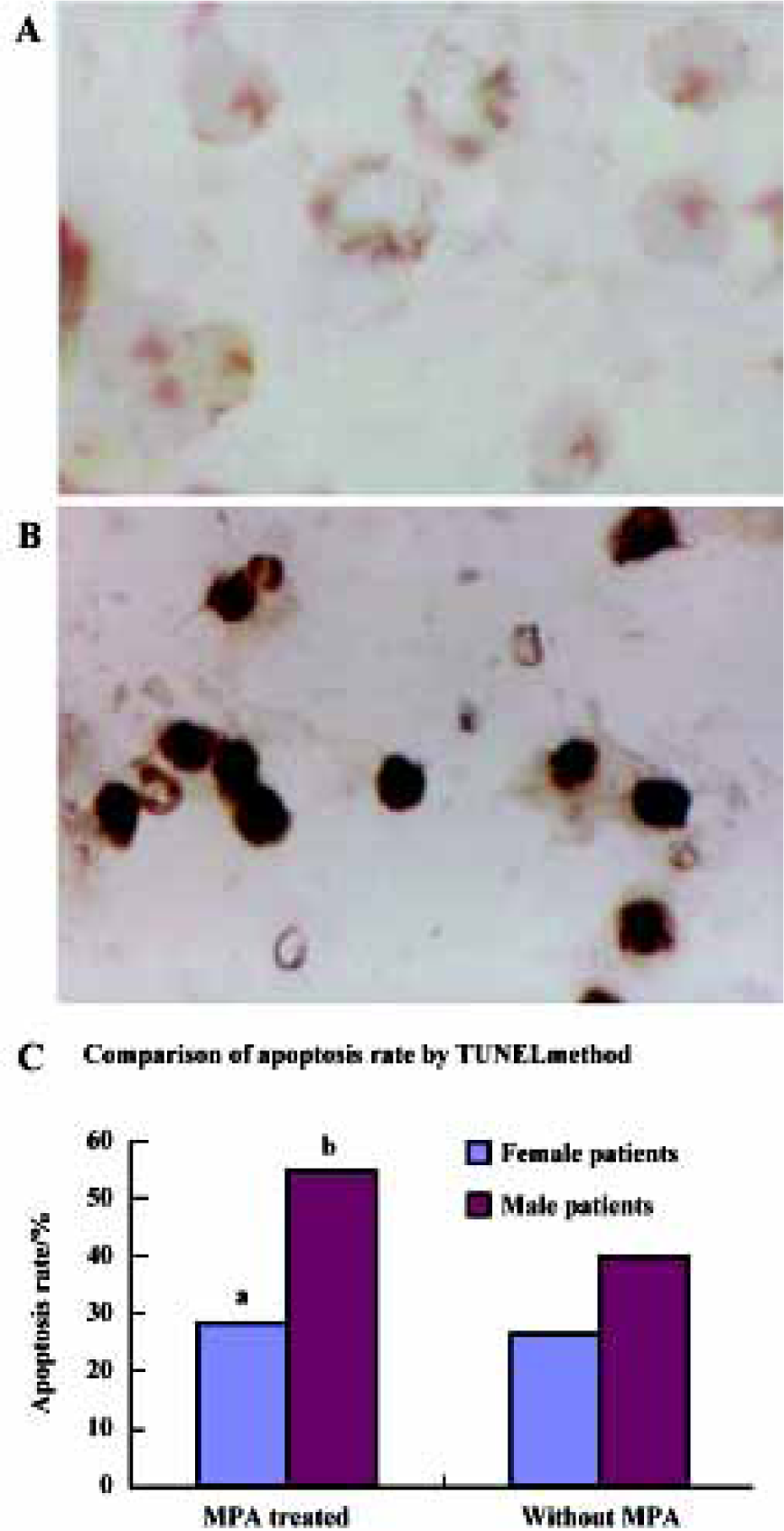
Effect of MPA on cell apoptosis After the peripheral blood mononuclear cells isolated from the patients were incubated with MPA 0.1 μmol/L for 3 d, a number of TUNEL-positive mononuclear cells were observed in male patients, (P<0.05), indicating that the peripheral blood mononuclear cells underwent apoptosis. The difference in apoptosis rate in female patients before and after MPA treatment was not significant (P>0.05) (Figure 1).
Discussion
ITP is a common immune disorder caused by platelet-reactive autologous antibodies. In some patients, platelet production is decreased as well. ITP in adults does not generally remit spontaneously, and most patients need hemostasia therapy. Corticosteroids, danazol, immuno-globulin, anti-D antibody, and several other agents inhibit clearance of the antibody-coated platelets, but the effect is not sufficient. Most patients will sustain a hemostatic response after splenectomy, although relapses can occur at any time[8]. Refractory idiopathic thrombocytopenic purpura represents a life-threatening condition that fails to respond to a variety of therapeutic measures[9]. In recent years, immunosuppressive agents such as cyclosporine A and MMF were used to treat refractory ITP as second line drugs. However, cyclosporine A has apparent side effects including hypertension, headache, and muscle pain. Thus, splenectomy could not be avoided, but could only be postponed in refractory ITP patients after they received cyclosporine A treatment. Howard et al reported that 4 patients with auto-immune haemolytic anemia and 5 of the 6 patients with auto-immune thrombocytopenia purpura showed a complete or good partial response to MMF[10], confirming the beneficial effects of MMF on refractory ITP. However, only 6 patients participated in the study. In the present study, MMF treatment was sustained for at least 1 month. Sixteen of the 20 (80%) patients had responses to MMF treatment; 9 (45%) achieved a complete response, 4 (20%) achieved a partial response, and 3 (15%) achieved minor response. The curative rate was 80%. The results indicated that MMF could be used as a second line agent for the treatment of refractory ITP. We also found that men achieved better response to MMF than women which was not been reported in other published studies[12]. The cell apoptosis rate was consistent with this conclusion. In long-term clinical observations, we also found that female ITP patients did not achieve better responses than male patients. We speculated that the difference might be related to different hormone levels and different MMF metabolization. However, the mechanism requires further study. MMF selectively inhibited the proliferation and survival of lymphocytes by inducing apoptosis and suppressing glycosylation and expression of adhesion molecules such as P-selectin, etc., which were over-expressed in ITP[13]. Our result that MPA induced apoptosis of PB lymphocytes from ITP patients in vitro was consistent with a previous report[14].
In contrast to MMF, we found that prednisone caused a significant increase in the number of myocarditis lesions. This is consistent with earlier studies that corticosteroids increased the severity of the disease during the acute phase of viral myocarditis in murine models[15]. In conclusion, long-term therapy with a median-dose of MMF is valuable for the treatment of refractory ITP. Randomized clinical trials need to be performed in the future.
References
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med 2002;346:995-1008.
- Bussel JB. Novel approaches to refractory immune thrombocytopenic purpura. Blood Rev 2002;16:31-6.
- Becker BN. Mycophenolate mofetil. Transplant Proc 1999;31:2777-8.
- Glander P, Hambach P, Braun KP, Fritsche L, Waiser J, Mai I, et al. Effect of mycophenolate mofetil on IMP dehydrogenase after the first dose and after long-term treatment in renal transplant recipients. Int J Clin Pharmacol Ther 2003;41:470-6.
- Gellermann J, Querfeld U. Frequently relapsing nephrotic syn-drome: treatment with mycophenolate mofetil. Pediatr Nephrol 2004;19:101-4.
- Wenzl HH, Hinterleitner TA, Aichbichler BW, Fickert P, Petritsch W. Mycophenolate mofetil for Crohn’s disease: short-term efficacy and long-term outcome. Aliment Pharmacol Ther 2004;19:427-34.
- Srinivas TR, Kaplan B, Meier-Kriesche HU. Mycophenolate mofetil in solid-organ transplantation. Expert Opin Pharmaco-ther 2003;4:2325-45.
- Cines DB, McKenzie SE, Siegel DL. Mechanisms of action of therapeutics in idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol 2003;25 Suppl 1:S52-6.
- Narang M, Penner JA, Williams D. Refractory autoimmune thrombocytopenic purpura: responses to treatment with a recombinant antibody to lymphocyte membrane antigen CD20 (rituximab). Am J Hematol 2003;74:263-7.
- Howard J, Hofferand AV, Prentice HG, Mehta A. Mycophenolate mofetil for the treatment of refractory auto immune haemolytic anemia and auto immune thrombocytopenia purpura. Br J Haematol 2002;117:712-5.
- Kneitz C, Wilhelm M, Tony HP. Effective B cell depletion with rituximab in the treatment of autoimmune disease. Immuno-biology 2002;206:519-27.
- Hou M, Peng J, Shi Y, Zhang C, Qin P, Zhao C, et al. Myco-phenolate mofetil for the treatment of steroid resistant idiopathic thrombocytopenic purpura. Eur J Hematol 2003;70:353-7.
- Goldsmith D, Carrey EA, Edbury S, Smolenski RT, Jagodzinski P, Simmonds HA. Mycophenolate mofetil, an inhibitor of IMP dehydrogenase, causes paradoxical elevation of GTP in erythrocytes of renal transplant patients. Clin Sci (Lond) 2004;107:63-8.
- Cohn RG, Mirkovich A. Mycophenolic acid increases apoptosis lysosomes and lipid droplets in human lymphoid and monocytic cell lines. Transplantation 1999;68:411-8.
- Padalko E, Verbeken E, Matthys P, Aerts JL, Clercq ED, Neyts J. Mycophenolate mofetil inhibits the development of Coxsackie B3-virus-induced myocarditis in mice. BMC Microbiol 2003;3:25-8.
