GLB prevents tumor metastasis of Lewis lung carcinoma by inhibiting tumor adhesion actions1
Introduction
The ability to metastasis is the most fearsome aspect of cancer and most cancer deaths are the sequel of metastatic diseases rather than primary tumor growth. In order to form overt metastases, a cell must complete the metastatic cascade, a series of well-defined steps including local invasiveness, intravasation, circulation, adhesion and extravasation, survival, proliferation and angiogenesis[1]. Within this multistep process, adhesive ability of metastatic tumor cells is a critical factor in extravasation and formation of new tumor foci[2–6].
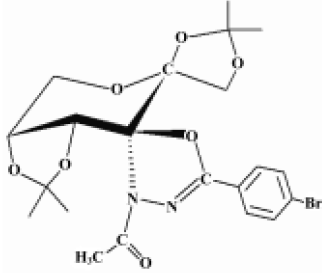
Cancer continues to represent the largest cause of mortality in the world and claims over 6 million lives every year[7]. An extremely promising strategy for the management of cancer today is chemoprevention, which is defined as the use of synthetic or natural agents (alone or in combination) to block the development of cancer in humans[8,9]. Studies on the pharmacological mechanisms and the search for new anticancer and anti-metastasis drugs are necessary and hold great interest for scientists. The compound GLB, (2''S,3aR,6S,7aR)-3''-acetyl-3a,7a-dihydro-2,2,2',2'-tetramethyl-5''-(4–bromophenyl)-spiro{spiro[1,3-dioxolo(4,5-D)-pyrane-6(7H),5'(4’H)-1',3'-dioxolo]-7(6H),2''(3''H)-(1,3,4)-oxadiazole} (Figure 1), was synthesized by Prof Zhong-jun LI (School of Pharmacy, Peking University) in 2000 and was patented by National Patent of China (N
Materials and methods
Cell culture Human umbilical vein endothelial cells (HUVEC) were isolated by collagenase I (1 g/L, Invitrogen, USA) digestion of umbilical veins from undamaged sections of fresh cords. HUVEC were grown in Medium 199 (Gibco, USA) supplemented with 20% heat-inactivated fetal bovine serum (FBS), 20 mg/L endothelial cell growth supplement (ECGS, Sigma, USA), 1×105 IU/L penicillin, 100 mg/L streptomycin and 2 mmol/L L-glutamine. Replicate cultures were obtained by trypsinization and cells of passages 3–6 were used in the experiments. The identification of HUVEC was confirmed by their polygonal morphology and by detecting their immunoreactivity for factor VIII-related antigens[10]. Human prostate carcinoma cells (PC-3M) were cultivated in RPMI-1640 containing 10% heat-inactivated FBS, 1×105 IU/L penicillin and 100 mg/L streptomycin in a humidified incubator with 5% CO2 in air at 37 °C.
Assay of PC-3M cell adhesion to HUVEC PC-3M cells were labeled with CFDA (carboxyfluorescein diacetate, Sigma, 100 mg/L) for 30 min at 37 °C[11]. Cells were washed twice with phosphate-buffered saline (PBS) to remove residual fluorescent dye. The viability of the cells was not compromised by this labeling protocol, as indicated by Trypan blue exclusion.
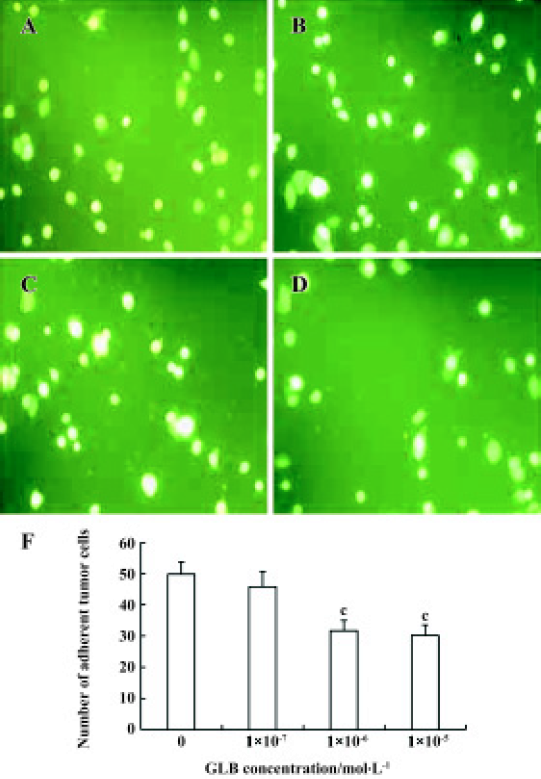
HUVEC were cultured to confluence in 96-well dishes coated with 2% gelatin. Confluence was confirmed using microscopy and CFDA-labeled PC-3M cells were washed and resuspended in culture medium. One hundred microliters of the tumor-cell suspension (about 1×104 cells) was added to HUVEC monolayers in 96-well dishes and co-cultured for 30 min at 37 °C. At the end of the experiment, the wells were washed twice with PBS to remove non-adhering PC-3M cells. The adherent cells were counted under a fluorescence microscope using an excitation wavelength of 492 nm, using five fields in each well[10].
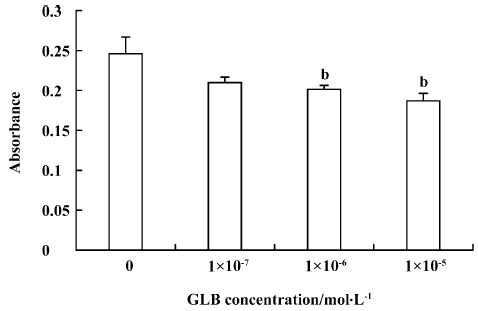
Assay of PC-3M cell adhesion to laminin The 96-well plates were coated with laminin (extracted by the Department of Cytobiology, Peking University) and incubated in RPMI-1640 containing 0.02% bovine serum albumin (BSA) for 15 min at 37 °C. In a total volume of 100 µL RPMI-1640, 8×104 PC-3M cells were added to each well and incubated for 4 h at 37 °C in the presence of GLB. After removing unattached cells with two washes with PBS, the attached cells were inccubated with 20 µL sterile MTT dye (Sigma) for a further 2 h at 37 °C. The medium was then removed, and 100 µL dimethyl sulfoxide (Me2SO) was added and mixed thoroughly. Spectrometric absorbance at 540 nm (for formazan dye) and 690 nm (as background level) was measured using a microplate reader (Bio-Rad, USA)[12].
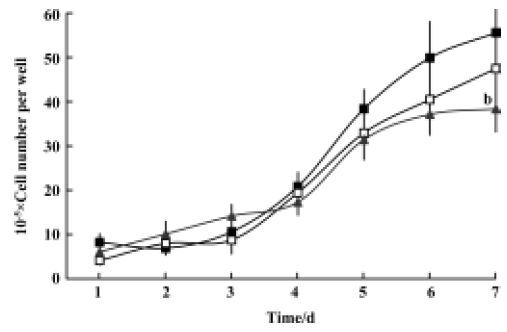
Proliferation assay PC-3M cells (1×104 cells/well) were cultured in 24-well plates and incubated for 1, 2, 3, 4, 5, and 6 d in the presence of GLB at various concentrations. The medium with GLB was changed every 48 h. After incubation the cells were harvested and washed with PBS. The number of viable cells in each well was then determined and counted using the Trypan blue exclusion assay[13].
Lewis lung carcinoma in vivo model Female C57BL/6 mice weighing 18 g–20 g were used and purchased from the Experimental Animal Center of Peking University (Grade II, Certificate N
Metastasis in vivo GLB was suspended in 0.5% (w/v) sodium carboxyl methylcellulose (CMC-Na) in distilled water and administered orally for 27 d (25, 50, and 100 mg·kg-1·d-1) from the next day of tumor cell injection. After the d 6, the tumor volumes of mice were measured every 3 d. At the d 28, the mice were killed and the lungs and primary tumors were removed and weighed. The number of metastasized pulmonary colonies was counted and lung tissues were fixed in formalin for further analysis. The inhibitory rate of lung metastasis (%) was calculated using the equation (Wmodel–Wtreatment)/(Wmodel–Wcontrol)×100%, where W is lung wet weight[14–16].
Pathological evaluation of lung The fixed lung tissue samples were embedded in paraffin, sectioned at 5 µm and stained with hematoxylin and eosin to evaluate alveolar integrity by counting area percentage of alveolus in lung. A total of 5 fields from each lung sample were screened randomly; the mean value was accepted as representative of the sample[17].
Statistical analysis The experimental results were expressed as mean±standard deviation (SD). Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Dunnett’s test with SPSS version 10.0. P<0.05 was considered to be statistically significant.
Results
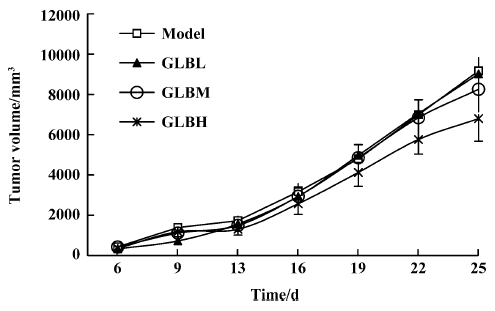
Inhibitory effects of GLB on spontaneous metastasis of Lewis lung carcinoma in mice We investigated the effect of oral administration of GLB on the spontaneous metastasis of Lewis lung carcinoma. GLB at the dose of 100 mg/kg significantly reduced the number of pulmonary metastatic colonies of Lewis lung carcinoma cells (P<0.05). The inhibitory rate of colony formation was approximately 31%. During the drug treatment, we found neither body weight loss nor toxic death in the GLB-treated mice. In addition to a reduction in the number of lung colonies by GLB, the survival rate of mice treated with GLB was significantly higher than that of untreated mice bearing Lewis lung carcinoma (Table 1). Although the mean weight and volume of the primary tumor in GLB-treated mice were less than those of vehicle-treated mice, there was no statistical difference (P>0.05, Table 1, Figure 2).
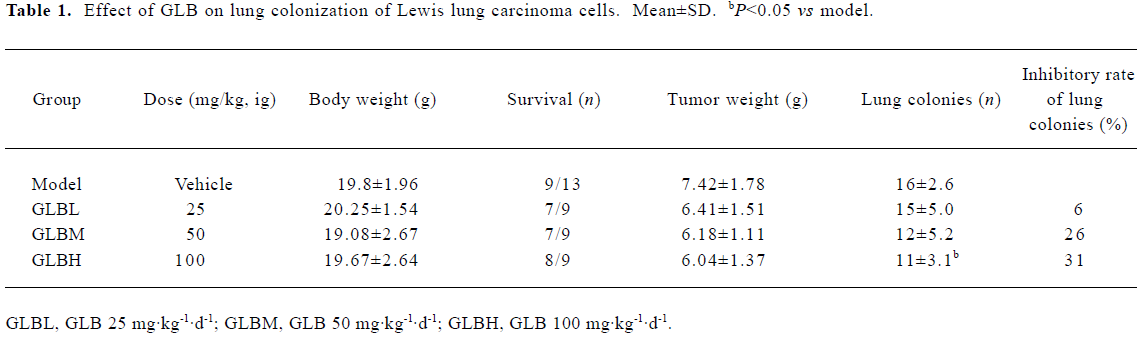
Full table
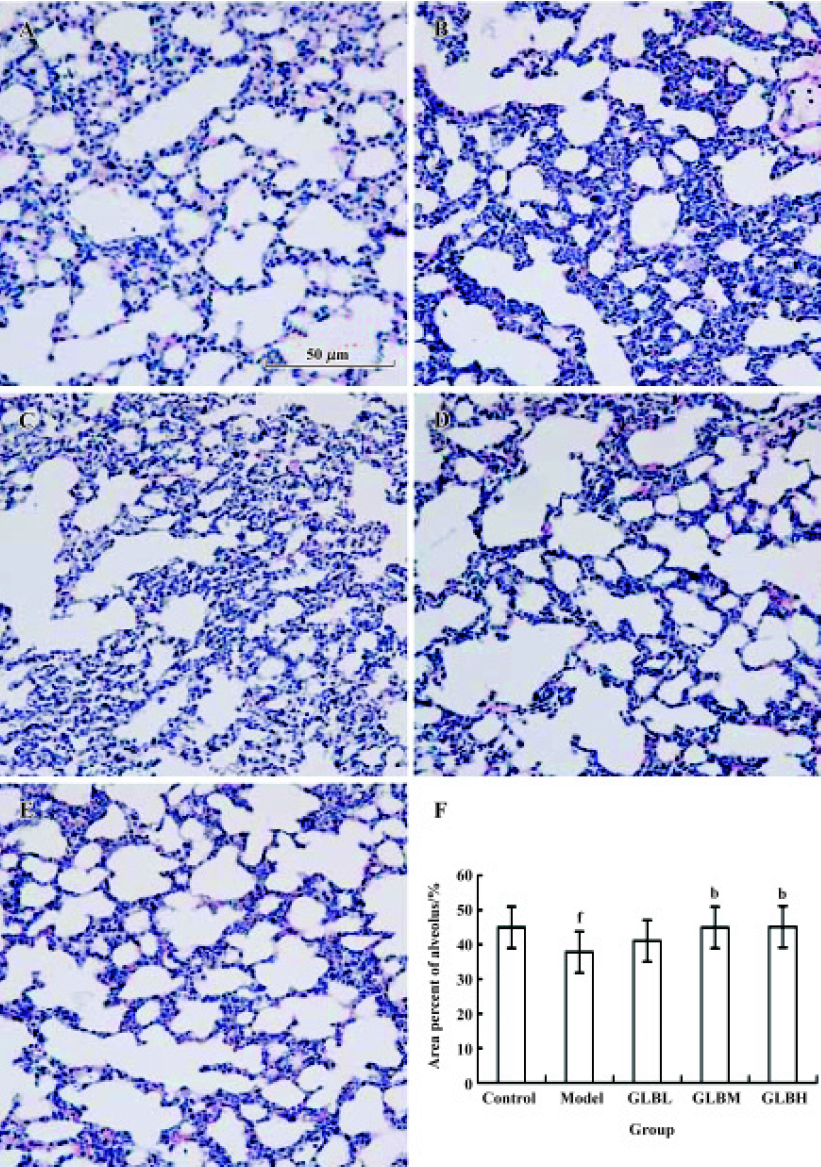
Effect of GLB on alveolar integrity in mice with Lewis lung carcinoma cells Alveolar integrity of lungs with metastatic tumor deposits in mice bearing Lewis lung carcinoma cells was significantly worse than that of normal lung tissue (area percentage of alveolus in lungs: 37.8%±7.44% vs 44.9%±7.6%, P<0.05). The lung tissue of mice treated with 100 mg/kg GLB was clearly better than that of tumor transplanted mice (area percentage of alveolus in lungs: 45.1%±6.13% vs 37.8%±7.4%, P<0.05) (Figure 3).
Inhibitory effects of GLB on metastatic adhesion function of tumor cells We evaluated the effects of GLB on the metastatic adhesion functions of tumor cells by examining its effect on PC-3M cell adhesion to endothelial monolayers and laminin. GLB, at the concentrations of 1×10-5 mol/L and 10-6 mol/L, inhibited significantly the adhesion of PC-3M cells to HUVEC (P<0.01, Figure 4). GLB at these concentrations also inhibited the attachment of PC-3M cells to the laminin (P<0.05, Figure 5). To clarify whether the inhibitory action on adhesion function could be due to a cytotoxic effect, GLB was tested for its effect on PC-3M cell proliferation (Figure 6). GLB had no substantial effect on the growth of tumor cells after up to 5 d incubation. Until the latter period of cell growth (d 6 and 7), the proliferation of PC-3M cells treated with 1×10-5 mol/L and 1×10-6 mol/L GLB showed signs of a slight decrease.
Discussion
As has been demonstrated, the sequential process of micrometastasis formation by Lewis lung carcinoma includes single cells in the blood circulation escaping from the immune system, adhering to epithelial cells, migrating into the tissue, proliferating and making colonies in the lung[18]. Investigate the earliest events during micrometastasis formation is useful and could be simply applied for the estimation of anti-metastatic and anti-adhesive effects of various anticancer agents[12].
The antitumor activities of the derivatives of oxadiazoles, one-membered heterocycles, have been reported and are found to be related to their chelating abilities or non-polarity, which is essential to penetrate through intracellular sites[19]. These include the new 5-(2-amino-3-pyridyl)-2-thioxo-3H-1,3,4-oxadiazole derivatives that have shown cytotoxic activity against the cells of 4 human tumor cell lines[20] and 2,5-disubstituted-1,3,4-oxadiazole compounds, which are potential anticancer agents[21]. The present study demonstrated that oral administration of GLB inhibited the spontaneous pulmonary metastasis of tumor cells in C57BL/6 mice and increased the survival rate. GLB significantly inhibited the adhesion by PC-3M cells without affecting cell proliferation in vitro. The present data suggest that GLB prevents tumor metastasis partly by inhibiting the metastatic adhesion of tumor cells.
Tumor cell adhesion to endothelial monolayers is a critical step in tumor metastasis[22], as is laminin expression in endothelial cells, being one of the earliest extracellular matrix (ECM) proteins. Laminin interaction with the β-1 integrin has been shown to have an important role in the regulation of tumor-to-endothelial cell adhesion[10]. We demonstrated that GLB significantly inhibited the adhesion of tumor cells to laminin, using the same doses of GLB as had been found to inhibit adhesion of tumor cells to endothelial cells. This suggests that the anti-adhesion activity of GLB might be due to its effect on laminin, one of many other factors involved in adhesion.
β-1 Integrin is known to recognize many ECM proteins as ligands, such as laminin, collagen and fibronectin. Laminin has been identified as the most important of these ligands for β-1 integrin in the cell adhesion process[23,24]. Whether GLB exerts its function through β-1 integrin or not is yet to be determined.
In the present study, GLB showed no effect on the weight of the primary tumor site in C57BL/6 mice subcutaneously injected with a Lewis lung carcinoma tumor cell suspension (Table 1). In a preliminary experiment where S180 carcinomas were implanted subcutaneously into ICR and LACA mice, GLB-treated mice (50 mg·kg-1·d-1 for 12 d, ip) showed a significant decrease in tumor weight, with inhibitory rates of 43% and 32% (tumor weight at d 12, mean±SD: 1.16±0.67 g vs 2.05 ± 0.64 g, P<0.01 for ICR mice; 1.81±0.68 g vs 2.65±0.81 g, P<0.05 for LACA mice; n=10 mice). The different effects of GLB on these in vivo assay models may be dependent on the kind of tumor, the period of the experiment and route of administration. We speculate that there might be other mechanisms of action for GLB that require further study, including the relationship between its effects on tumor growth or metastasis and its inhibition of angiogenesis.
In conclusion, the compound GLB prevents tumor metastasis by inhibiting tumor adhesion. This suggests it has potential for future anti-metastatic development.
References
- Gassmann P, Enns A, Haier J. Role of tumor cell adhesion and migration in organ-specific metastasis formation. Onkologie 2004;27:577-82.
- Nicolson GL. Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Curr Opin Cell Biol 1989;1:1009-19.
- Lewalle JM, Cataldo D, Bajou K, Lambert CA, Foidart JM. Endothelial cell intracellular Ca2+ concentration is increased upon breast tumor cell contact and mediates tumor cell transendothelial migration. Clin Exp Metastasis 1998;16:21-9.
- Honn KV, Tang DG. Adhesion molecules and tumor cell interaction with endothelium and subendothelial extracellular matrix. Cancer Metastasis Rev 1992;11:353-75.
- Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, et al. Steps in tumor metastasis: new concept for intravital videomicroscopy. Cancer Metastasis Rev 1995;14:279-301.
- Tressler RJ, Yeatman T, Nicolson GL. Extracellular annexin VI expression is associated with divalent cation-dependent endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. Exp Cell Res 1994;215:395-400.
- Abdullaev FI, Luna RR, Roitenburd BV, Espinosa AJ. Pattern of childhood cancer mortility in Mexico. Arch Med Res 2000;31:526-31.
- Gupta M, Mazumder UK, Kumar RS, Kumar TS. Antitumor activity and antioxidant role of Bauhinia racemosa against Ehrlich ascites carcinoma in Swiss albino mice. Acta Pharmacol Sin 2004;25:1070-6.
- Rao KVK, Schwartz SA, Nair HK, Aalinkeel R, Mahajan S, Chawda R, et al. Plant derived products as a source of cellular growth inhibitory phytochemicals on PC-3M, DU-145 and LNCaP prostate cancer cell lines. Curr Sci 2004;87:1585-8.
- Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of β-1 integrin expression. J Surg Res 2001;97:14-9.
- Woodward JK, Nichols CE, Rennie IG, Parsons MA, Murray AK, Sisley K. An in vitro assay to assess uveal melanoma invasion across endothelial and basement membrane barriers. Invest Ophthalmol Vis Sci 2002;43:1708-14.
- Yoshitomi Y, Nakanishi H, Kusano Y, Munesue S, Oguri K, Tatematsu M, et al. Inhibition of experimental lung metastases of Lewis lung carcinoma cells by chemically modified heparin with reduced anticoagulant activity. Cancer Lett 2004;207:165-74.
- Tozawa K, Kawai N, Hayashi Y, Sasaki S, Kohri K, Okamoto T. Gold compounds inhibit adhesion of human cancer cells to vascular endothelial cells. Cancer Lett 2003;196:93-100.
- Xiang Y, Ma B, Li T, Yu HM, Li XJ. Acetazolamide suppresses tumor metastasis and related protein expression in mice bearing Lewis lung carcinoma. Acta Pharmacol Sin 2002;23:745-51.
- Prontera C, Mariani B, Rossi C, Poggi A, Rotilio D. Inhibition of gelatinase A (MMP-2) by batimastat and captopril reduces tumor growth and lung metastases in mice bearing Lewis lung carcinoma. Int J Cancer 1999;81:761-6.
- Ma B, Xiang Y, Li T, Yu HM, Li XJ. Inhibitory effect of topiramater on Lewis lung carcinoma metastasis and its relation with AQP1 water channel. Acta Pharmacol Sin 2004;25:54-60.
- Ustundag N, Bozkurt AK, Demirkaya A, Koksal C, Mayda AS. Histopathological and immunohistochemical detection of protective effects of University of Wisconsin solution supplemented with iloprost on donor lung damage. Transplant Proc 2004;36:1271-4.
- Hirano S. In vitro and in vivo cytotoxic effects of nitric oxide on metastatic cells. Cancer Lett 1997;115:57-62.
- Mishra L, Said MK, Itokawa H, Takeya K. Antitumor and antimicrobial activities of Fe(II)/Fe(III) complexes derived from some heterocyclic compounds. Bioorg Med Chem 1995;3:1241-5.
- Liszkiewicz H, Kowalska MW, Wietrzyk J, Opolski A. Synthesis and anti-proliferative activity of new 5-(2-amino-3-pyridyl)-2-thioxo-3-1,3,4-oxadiazole derivatives. Indian J Chem, B 2003; 42B: 2846–52.
- Shah HP, Shah BR, Bhatt JJ, Desai NC, Trivedi PB, Undavia NK. Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles as potential antimicrobial, anticancer and anti-HIV agents. Indian J Chem, B 1998; 37B: 180–2.
- Danen EH, Marcinkiewicz C, Cornelissen IM, van Kraats AA, Pachter JA, Ruiter DJ, et al. The disintegrin eristostatin interferes with integrin a4b1 function and with experimental metastasis of human melanoma cells. Exp Cell Res 1998;238:188-96.
- Dejana E. Endothelial cell adhesive receptors. J Cardiovasc Pharmacol 1993;21:S18-21.
- Kitayama J, Nagawa H, Tsuno N, Osada T, Hatano K, Sunami E, et al. Laminin mediates tethering and spreading of colon cancer cells in physiological shear flow. Br J Cancer 1999;80:1927-34.