Pioglitazone can ameliorate insulin resistance in low-dose streptozotocin and high sucrose-fat diet induced obese rats
Introduction
Insulin resistance is a major characteristic of various metabolic disorders, including type 2 diabetes and obesity[1]. There is strong evidence that an important factor leading to obesity and insulin resistance is an increase in energy intake[2]. Our previous study demonstrated that high-fat, high-sucrose feeding led to insulin resistance and impaired glucose tolerance in female Wistar rats[3]. In our study, female rats were given a relatively low-dose of streptozotocin (STZ), followed by a high sucrose-fat diet containing 52% sucrose, 24% fat, and 18% protein (4.8 cal/g chow). Compared to the control group, which did not receive either STZ or a high sucrose-fat diet, the treatment group showed obvious obesity and impaired glucose tolerance after 8 weeks. Fasting blood glucose levels increased slightly or moderately, and were accompanied by hyperinsulinemia, dyslipidemia, enhanced gluconeogenesis, and reduced insulin tolerance. These characteristics indicated insulin resistance and obesity with mild β-cell dysfunction, which was further induced by the high sucrose-fat diet. A similar result is observed in humans. Our results suggested that this model was a successful insulin-resistant rat model and is useful in the study of insulin resistance and insulin sensitivity.
Thiazolidinediones (TZDs), a new class of insulin sensitizing drugs including troglitazone, pioglitazone, and rosiglitazone, provide an effective approach for treating type 2 diabetes. TZDs improve insulin sensitivity, impair glucose tolerance and dyslipidemia in type 2 diabetics[4], as well as in obese non-diabetic subjects[5]. Similar findings have been demonstrated in a number of genetic and non-genetic animal models of diabetes/insulin resistance[6,7]. TZDs elicit their effects through activating the peroxisome proliferator–activator receptor (PPAR)-γ, a nuclear hormone receptor. When ligands stimulate the PPAR-γ nuclear receptor, a variety of response genes are stimulated or repressed. The TZD PPAR-γ agonists improve insulin sensitivity via multiple mechanisms. Although the exact target genes for insulin sensitization remain unknown, it has been demonstrated in vitro and in vivo that treatment with TZDs affects a variety of factors involved in lipid metabolism, insulin signal pathways, glucose phosphorylation, and glucose transport.
In the present study, we evaluated the effect of pioglitazone, a PPAR-γ agonist, on insulin resistance and related abnormalities in low-dose STZ and high sucrose-fat diet induced obese rats. Our findings demonstrated that PPAR-γ agonist treatment reduced circulating and stored lipids and improved the insulin-resistant status in model rats.
Materials and methods
Animals Thirty female Wistar rats, weighing 225–250 g, were obtained from the Experimental Animal Center, Chinese Academy of Medical Sciences, Beijing, China [Certificate N
After 8 weeks on a high sucrose-fat diet, weight-matched, fasting-blood-glucose-matched STZ rats were randomly divided into two groups, a STZ group and a pioglitazone group. STZ rats (n=8) were given vehicle solution and pioglitazone rats (n=7) were orally administered pioglitazone (20 mg·kg-1·d-1; the pioglitazone was a gift from Prof Yu-ling LIU) as a suspension in 1% Tween 80 solution (the high sucrose-fat diet was given to the rats during this period). Rats in each group were treated for 28 d. Glucose tolerance tests, insulin tolerance tests and gluconeogenesis tests were carried out over the last 14 d of the experiment. At the end of the treatment period, all animals were decapitated after a 4-h fast. Blood samples were collected and serum was prepared and kept at -20 ºC for the determination of serum lipids and immunoreactive insulin. Celiac fats were excised for weighing. Skeletal muscles and livers were separated and frozen immediately in liquid nitrogen, and stored at -70 ºC for further analysis of GLUT4 and IRS-1.
Biochemical analysis Serum glucose levels were assayed using the glucose oxidase method. Serum insulin was determined using a radioimmunoassay kit (China Institute of Atomic Energy, Beijing, China). Free fatty acid (FFA) concentrations were measured using the Cu2+ reagent method. Triglyceride (TG), total cholesterol (TC), and HDL-cholesterol (HDL-C) levels were determined using the colorimetric method with commercial kits (Zhong sheng bei kong Bio-Technology and Science, Beijing, China).
Intraperitoneal glucose tolerance test (IPGTT) Baseline glucose levels were determined after a 4-h fast, after which glucose (2 g/kg body wt) was administered intraperitoneally to non-sedated animals. Tail vein blood was sampled for glucose determination 30 and 120 min after glucose administration.
Insulin tolerance test (ITT) Baseline glucose levels were determined after a 4-h fast. Insulin (0.4 U/kg body wt) was injected subcutaneously. Blood was sampled from the tail tip 40 and 90 min after insulin administration, and the glucose concentration was determined. Insulin injections and blood glucose sampling took approximately the same amount of time per animal (ie 25 animals were injected in 12 min and blood glucose sampling of those same 25 animals also took approximately 12 min), so that the sample times are accurate for each animal.
Gluconeogenesis test Baseline glucose levels were determined after an overnight fast. DL-alpha alanine was injected intraperitoneally. Blood was sampled from the tail and glucose levels were determined at 0 and 60 min. The percent increase in glucose level at 60 min was calculated. Higher values indicated higher levels of gluconeogenesis.
GLUT4 and IRS-1 protein expression Protein extractions and immunoblots for the determination of GLUT4 were carried out on frozen skeletal muscle from 12 rats using a modified Klip’s method[8]. Skeletal muscle (1 g) was powdered under liquid nitrogen and homogenized for 20 s in buffer (pH 7.4) containing sucrose 250 mmol/L, Tris 50 mmol/L and edetic acid 0.2 mmol/L. The homogenates were centrifuged at 9000×g for 10 min (4 ºC) and the supernatants were reserved. The pellet was cleaned with buffer and centrifuged three times. All three supernatants were mixed and centrifuged at 190 000×g for 60 min (4 ºC). The resulting pellet was resuspended in a small amount of buffer (about 0.5 mL) as a total membrane fraction. Protein concentrations of the suspensions were determined using the Coomasine brilliant blue method[8] prior to Western blot analysis.
Frozen tissues (100 mg liver, 100 mg skeletal muscle) were ground into a fine powder with a mortar and pestle and homogenized in the buffer (1% Triton X-100, sodium pyrophosphate 100 mmol/L, HEPES 50 mmol/L, pH 7.4, NaF 100 mmol/L, PMSF 2 mmol/L, 0.1% aprotinin, sodium edetic acid 10 mmol/L, Na3VO4 10 mmol/L) with a polytron homogenizer at 4 ºC for 6 (for muscle) or 10 (for liver) times[9,10]. The homogenates were centrifuged at 10 000×g at 4 ºC for 60 min and the supernatants were collected for IRS-1 analysis.
Protein extracts were resuspended in Laemmli buffer and separated using sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% (for GLUT4) or 7.5% (for IRS-1) polyacrylamide gels. Proteins were electrophoretically transferred to nitrocellulose membranes. The nitrocellulose membranes were incubated in phosphate buffer saline contained 0.1% Tween-20 overnight at 4 ºC to reduce non-specific binding and blotted with GLUT4 (anti-rabbit carboxy-terminal GLUT4, Santa Cruz Biotechnology, California, USA 1:1000) and IRS-1 (anti-rabbit carboxy-terminal IRS-1, Santa Cruz Biotechnology) antibodies according to the manufacturer’s instructions. After incubation with peroxidase-conjugated secondary antibodies (1:2500, Santa Cruz Biotechnology), proteins were visualized using enhanced chemiluminescence. Band intensities were quantified by densitometry.
Statistical analysis The values presented are expressed as mean±SD. Statistical analyses were carried out using ANOVA. P<0.05 was considered to be statistically significant.
Results
Effect of pioglitazone on body weight, adipose weight, fasting blood glucose (FBG), fasting blood insulin (FBI), and insulin sensitivity index (ISI) STZ rats had significantly elevated levels of glucose and insulin compared with control rats (P<0.01) (Table 1). Reduced ISI (1/FBG×FBI) values revealed that insulin resistance developed in STZ rats. Treatment with pioglitazone for 28 d significantly lowered fasting glucose and insulin levels, and improved insulin sensitivity. STZ rats had significantly increased body weight and mass of celiac fat (P<0.01). There was no significant difference in body weight and adipose tissue weight between STZ animals and pioglitazone-treated animals (Table 1).
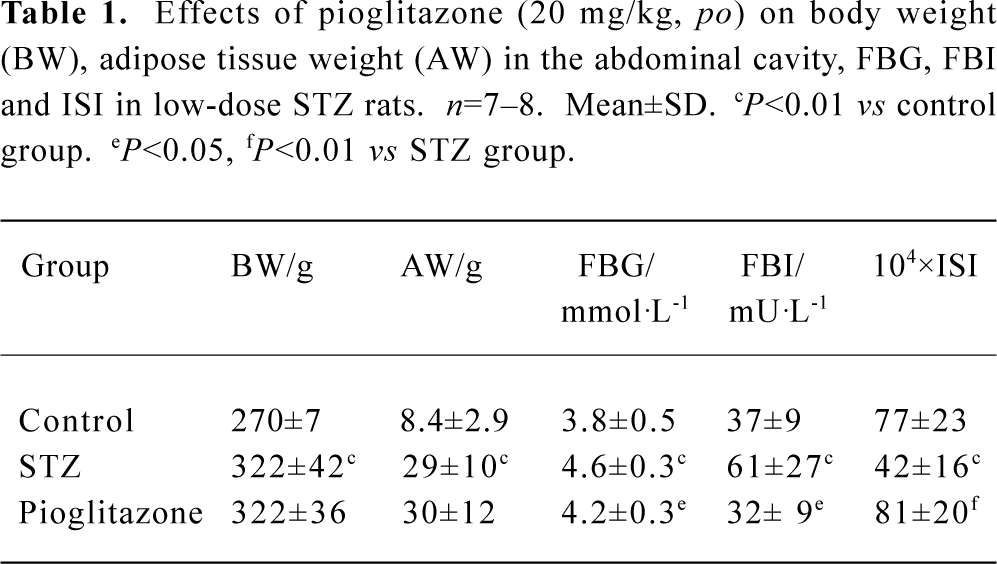
Full table
Effect of pioglitazone on glucose tolerance in STZ rats The IPGTT results revealed that rats in the STZ group had significantly impaired glucose tolerance compared with those in the control group. Surprisingly, the area under the curve (AUC) indicated that no effect attributable to pioglitazone administration on glucose tolerance was observed in the pioglitazone-treated group, although fasting glucose was slightly but significantly decreased (Table 2).
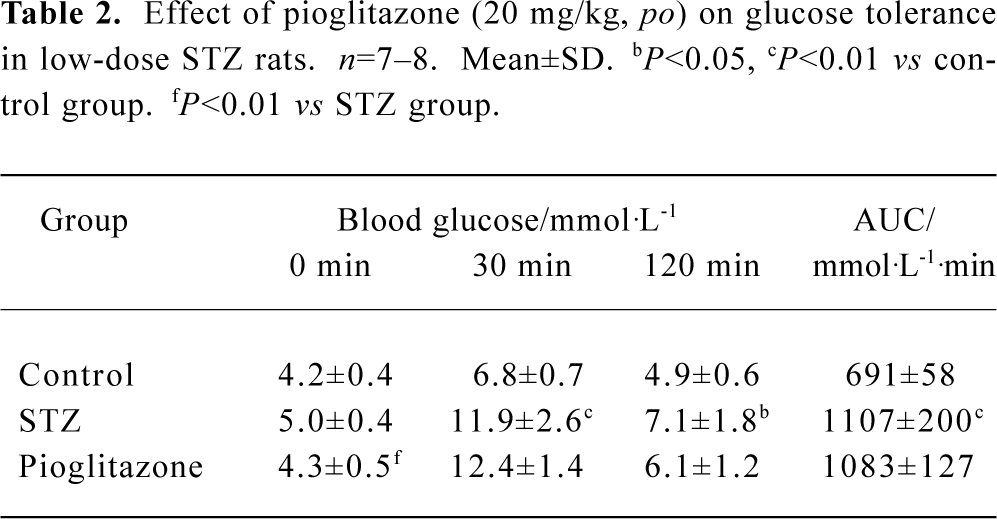
Full table
Effect of pioglitazone on insulin tolerance in STZ rats The results of the ITT showed that insulin resistance had developed significantly in STZ rats. Treatment with pioglitazone for 19 d markedly decreased glucose levels at every point, including AUC, indicating that insulin sensitivity was improved. In particular, the data suggested a prolongation in insulin action (Table 3).
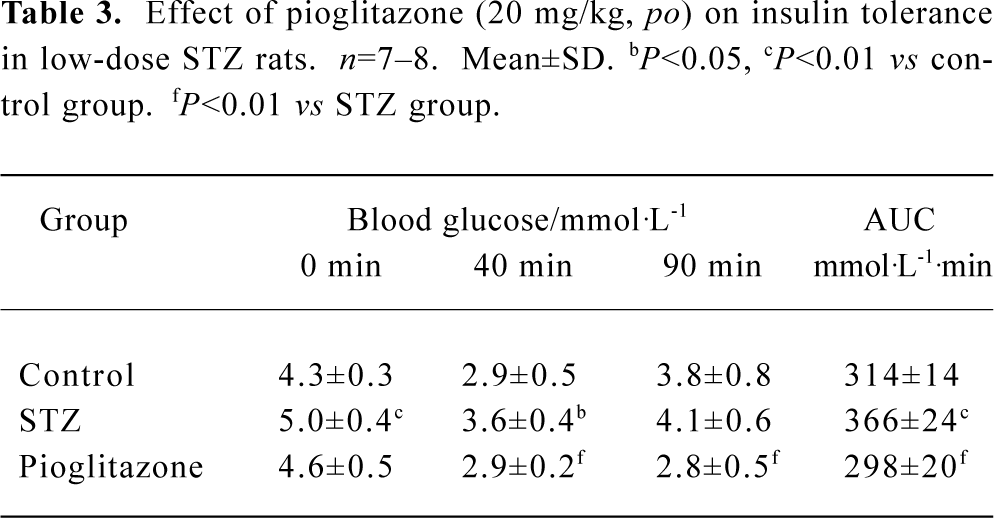
Full table
Effect of pioglitazone on gluconeogenesis in STZ rats Under conditions of insulin resistance, gluconeogenesis from non-glucose substrates was always elevated in STZ rats. Our results showed that pioglitazone significantly reduced glucose production from alanine, which was assessed by a percentage increase in blood glucose levels at 60 min (Table 4).
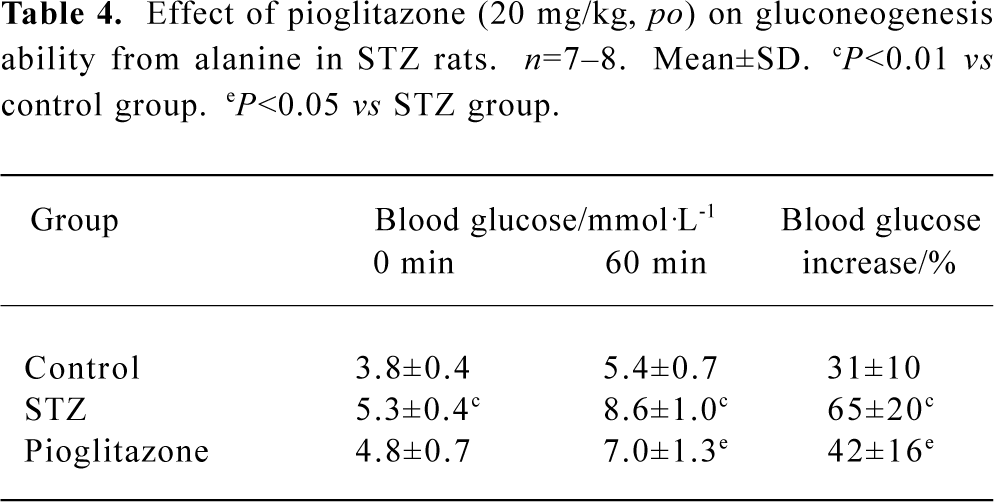
Full table
Effect of pioglitazone on lipid profiles in serum, liver, and muscle The changes in lipid contents in these three groups (Table 5) indicated that the serum TG, TC, and FFA levels were all elevated significantly in the untreated STZ group, whereas HDL-C decreased. Pioglitazone treatment decreased TG, TC, and FFA and increased HDL-C levels significantly. Elevated liver TG, TC, FFA levels and muscle TG were observed in STZ rats and were normalized by pioglitazone treatment. These findings may indicate that tissue lipid levels are closely associated with insulin sensitivity. The TC/HDL-C ratio is considered to be a predictor of cardiovascular disease (CVD). TC/HDL-C values suggested that pioglitazone diminished the potential risk of CVD by correcting dyslipidemia.
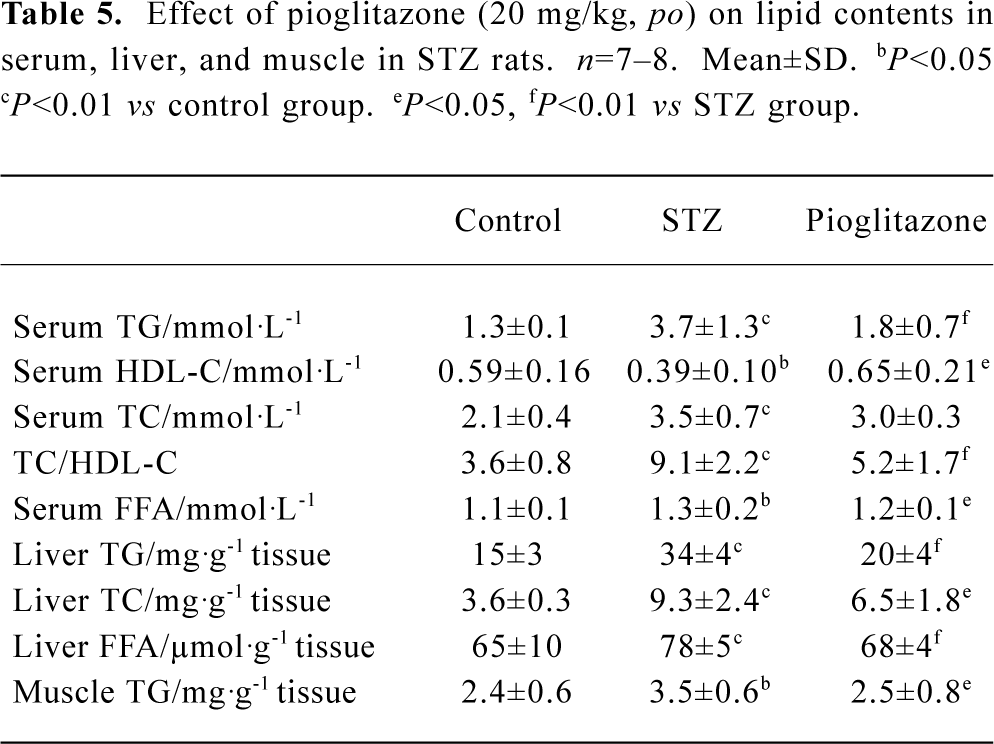
Full table
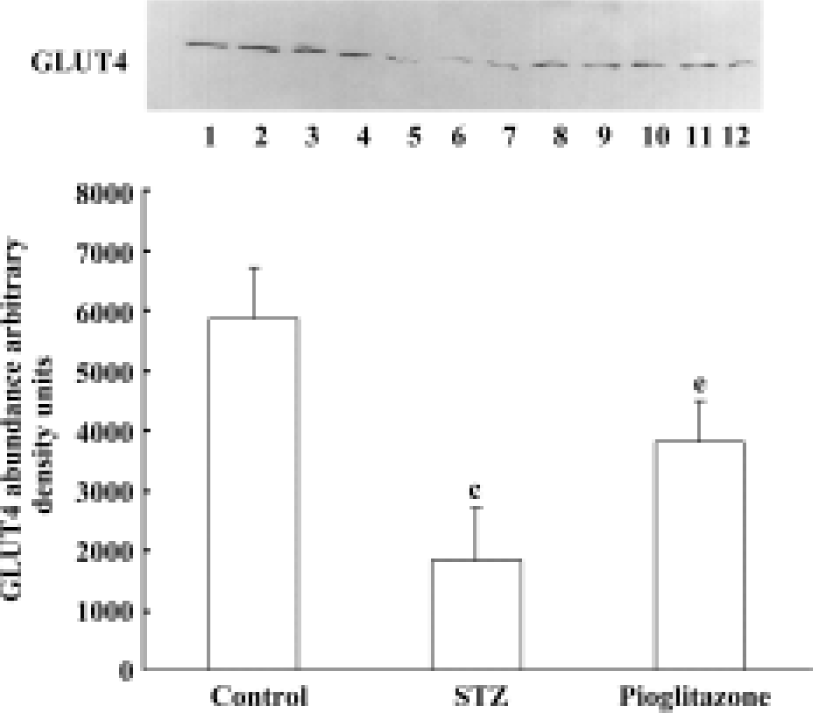
Effect of pioglitazone on GLUT4 content in skeletal muscle membrane Membrane GLUT4 protein content was decreased by 68.7% in skeletal muscles from untreated STZ rats compared with control rats (Figure 1). Pioglitazone treatment increased GLUT4 protein levels significantly.
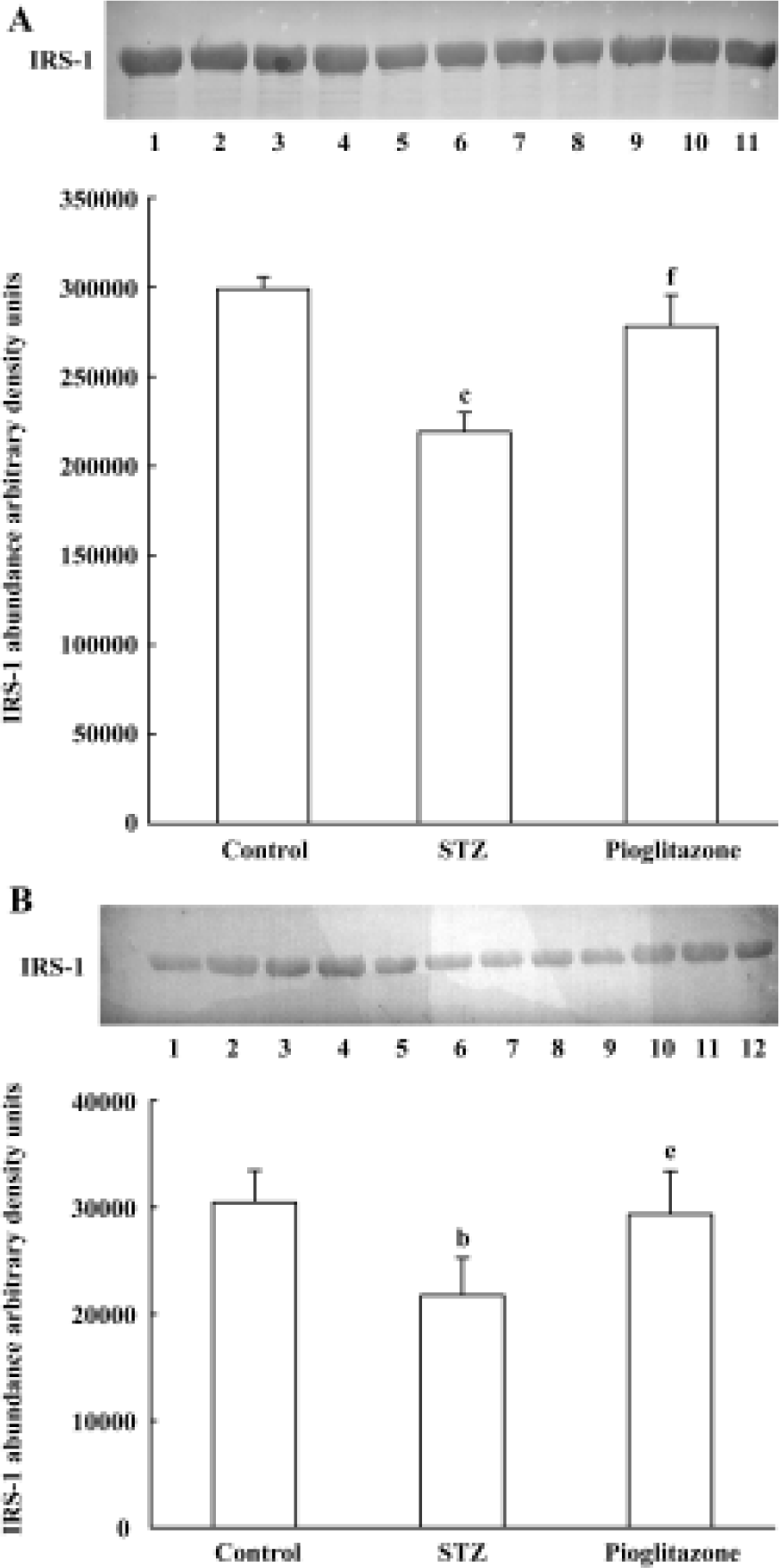
Effect of pioglitazone on IRS-1 expression in liver and skeletal muscle Statistically significant (A, 26.7%; B, 28.6%) decreases in IRS-1 proteins were observed in untreated STZ rats compared with control rats (Figure 2). Pioglitazone treatment for 28 d increased IRS-1 protein expression in liver and skeletal muscle significantly.
Discussion
Insulin resistance is a characteristic feature of type 2 diabetes and other pathophysiological states in humans. Therefore, amelioration of insulin sensitivity is an important therapeutic goal. Diet plays an important role in the development of insulin resistance and type 2 diabetes. An excessive intake of fat and sugar can lead to obesity and diabetes[11]. Previous studies have shown that high fat (59%) feeding for 24 d leads to significant insulin resistance. Euglycemic clamp revealed that glucose disposal rate (GDR) decreased by 50%; however, hyperglycemia was not observed[12]. Pascoe and Storlien reported that low-dose STZ (45 mg/kg body wt) administration to neonatal rats caused no change in glucose and insulin levels after 8 weeks; however, hyperglycemia and insulin resistance developed after high-fat feeding for 1 week[13]. In the present study, we injected STZ at low doses to Wistar rats to induce light damage of islet cells, leading to glucose intolerance. On this basis, a high sucrose-fat diet was followed to induce obesity. Our previous study reported that glucose infusion rate (GIR) decreased significantly in model rats compared with control rats (15.1±4.8 vs 27.3±2.9 mg/kg per min, P<0.01)[14]. This model is similar to the genetically insulin-resistant obese Zucker rats, which are characterized by a range of metabolic abnormalities including severe hyperinsulinemia, dyslipidemia and adipocyte hypertrophy; however, they possess normal blood glucose levels[15]. The precise mechanisms responsible for this defect remain unknown.
Major progress has been made in understanding the mechanisms that causally underlie the metabolic actions of TZDs. Although the exact mechanisms remain uncertain, it has been widely reported that TZDs activate PPAR-γ and stimulate adipocyte differentiation, which might modulate the glucose metabolism of other tissues.
In the present study, we investigated the effects of a particular TZD, pioglitazone, on insulin resistance induced by low-dose STZ and a high sucrose-fat diet in Wistar rats. The model rats showed significant elevation in fasting blood glucose and insulin levels, as well as in circulating TG, TC, and FFA levels. Pioglitazone treatment markedly normalized serum lipid contents and decreased lipid storage in insulin-sensitive tissues. In our previous study, a strong correlation was found between circulating triglycerides and in vivo insulin resistance as assessed by the hyperinsulinemic-euglycemic clamp. Evidence from other studies also suggests an important role for tissue lipid levels in insulin action[16]. It was, therefore, suggested that regulating lipid metabolism might play an important role in the insulin sensitizing effect of pioglitazone.
Studies in vitro have shown that a major effect of TZDs is the inhibition of gluconeogenesis in isolated hepatocytes[17]. Our gluconeogenesis test revealed that pioglitazone significantly inhibited the impaired gluconeogenesis in STZ rats.
Our study also shed some light on the potential molecular mechanisms underlying amelioration of insulin resistance. We focused on the insulin sensitive tissues, that is, liver and skeletal muscle. It has been reported that glucose transport decreases in the skeletal muscle of high-fat or high-sucrose induced insulin-resistant rats, and that GLUT4 mRNA and protein expression also decrease[18]. In insulin-deficient type 1 diabetic rats, GLUT4 expression also decreased[19]. Our results are consistent with these reports and show a 68.7% decrement in skeletal GLUT4 protein content in STZ rats compared with control rats, although we did not measure plasmid membrane GLUT4 content during insulin stimulation, which reflects GLUT4 translocation. Although it is unlikely that changes in GLUT4 content entirely explain the insulin sensitizing effects of pioglitazone treatment because of various defects in the intracellular insulin-signaling pathway, GLUT4 is contributing to a certain extent.
IRS-1 is a well-described insulin receptor substrate that, after tyrosine phosphorylation, is associated with and activates PI3-kinase, and plays an important role in insulin signaling[20]. Our results showed a marked deficiency in IRS-1 protein content in untreated STZ rats, with a significant improvement towards normal levels after pioglitazone treatment. Pioglitazone induced increases in GLUT4 and IRS-1 protein expression, which were associated with improvements in glucose transport and insulin signaling pathways, and this may explain the effects of pioglitazone in improving insulin resistance in the model animals.
In the present study, pioglitazone treatment improved insulin sensitivity as assessed by both ISI and ITT; however, pioglitazone had no effect on glucose intolerance in STZ rats. Glucose intolerance pre-existed prior to high sucrose-fat diet feeding, which means that the impaired glucose tolerance was originally caused by STZ administration, and developed gradually with feeding. The reasons for this remain uncertain and require further investigation, particularly the effects on β-cell function.
In conclusion, our studies showed a marked state of insulin resistance and obesity in STZ rats that is associated with various defects in glucose and lipid metabolism, including the insulin signaling–glucose transport pathway. Despite the persistence of obesity in these animals, pioglitazone treatment led to an apparent improvement in overall insulin sensitivity by ameliorating dyslipidemia, hyperinsulinemia, and gluconeogenesis, as well as affecting glucose transport and insulin signaling.
References
- Defronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992;15:318-68.
- Hallfrisch J, Lazar F, Jorgensen C, Reiser S. Insulin and glucose responses in rats fed sucrose or starch. Am J Clin Nutr 1979;32:787-93.
- Xie MZ, Liu HF, Zhang LY, Shen ZF, Chen QM. An experimental rat model of obesity and diabetes. Acta Pharm Sin 1985;20:801-6.
- Suter SL, Nolan JJ, Wallace P, Gumbiner B, Olefsky JM. Metabolic effects of new oral hypoglycemic agent CS-045 in NIDDM subjects. Diabetes Care 1992;15:193-203.
- Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med 1994;331:1188-93.
- Ikeda H, Taketomi S, Sugiyama Y, Shimura Y, Sohda T, Meguro K, et al. Effects of pioglitazone on glucose and lipid metabolism in normal and insulin resistant animals. Arzneimittelforschung 1990;40:156-62.
- Oakes ND, Camilleri S, Furler SM, Chisholm DJ, Kraegen EW. The insulin sensitizer, BRL 49653, reduces systemic fatty acid supply and utilization and tissue lipid availability in the rat. Metabolism 1997;46:935-42.
- Klip A, Ramlal T, Young DA, Holloszy JO. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett 1987;224:224-30.
- Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest 1994;94:1543-9.
- Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest 1993;92:2065-72.
- Perriello G, Misericordia P, Volpi E, Pampanelli S, Santeusanio F, Brunetti P, et al. Contribution of obesity to insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1995;80:2464-9.
- Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 1986;29:192-8.
- Pascoe WS, Storlien LH. Inducement by fat feeding of basal hyperglycemia in rats with abnormal β-cell function: model for study of etiology and pathogenesis of NIDDM. Diabetes 1990;39:226-33.
- Ding SY, Shen ZF, Chen YT, Xie MZ. Evaluation on two insulin resistance rat models by euglycemic clamp technique. Chin J Diabetes 2001;9:286-90.
- Terrettaz J, Assimacopoulos-Jeannet F, Jeanrenaud B. Severe hepatic and peripheral insulin resistance as evidenced by euglycemic clamps in genetically obese fa/fa rats. Endocrinology 1986;118:674-8.
- Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA 2001;98:7522-7.
- Ciaraldi TP, Gilmore A, Olefsky JM, Goldberg M, Heidenreich KA. In vitro studies on the action of CS-045, a new antidiabetic agent. Metabolism 1990;39:1056-62.
- Sevilla L, Guma A, Enrique-Tarancon G, Mora S, Munoz P, Palacin M, et al. Chronic high-fat feeding and middle-aging reduce in an additive fashion Glut4 expression in skeletal muscle and adipose tissue. Biochem Biophys Res Commun 1997;235:89-93.
- Garvey WT, Huecksteadt TP, Birnbaum MJ. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science 1989;245:60-3.
- Kanai F, Ito K, Todaka M, Hayashi H, Kamohara S, Ishii K, et al. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI-3 kinase. Biochem Biophys Res Commun 1993;195:762-8.
