Activation of human tonsil and skin mast cells by agonists of proteinase activated receptor-2
Introduction
Recently, it was summarized that apart from allergic disease, mast cells were associated with at least 35 different non-allergic clinical disorders[1]. The increased numbers of mast cells or mast cell degranulation being observed in these diseases implied that this cell type was most likely involved in the pathogenesis of these diseases. Since mast cells carry out their functions mainly through their released mediators including histamine, tryptase, chymase, heparin, cytokines, and other products[2,3], the understanding of mediator release properties of mast cells is crucial for our study on the roles of mast cells in diseases.
Tryptase is a tetrameric serine proteinase that constitutes some 20% of the total protein within human mast cells and is stored almost exclusively in the secretory granules of mast cells[4] in a catalytically active form[5]. Upon degranulation, tryptase is released from mast cells along with histamine and other mast cell products.
For more than four decades, histamine has been widely used as a marker of mast cell degranulation in vitro, and numerous anti-allergic drugs such as sodium cromoglycate, lodoxamide, salbutamal, ketotifen, terfenadine, and cetiri-zine[6,7], and salmeteral[8] were reported to inhibit anti-IgE induced histamine release from human tonsil, skin, or lung mast cells. Therefore, both tryptase and histamine were used as markers of mast cell degranulation in the current study.
In recent years, it was found that PAR-2, a receptor of trypsin and tryptase[9] was expressed on human mast cells[10] and PAR-2 agonists were reported to be capable of activating rat peritoneal mast cells[11] and human gut mast cells[12]. However, the potential effects of PAR-2 agonists including trypsin, SLIGKV, and tc-LIGRLO[13] on human tonsil and skin mast cells have not been examined. Therefore, the actions of these PAR-2 agonists on tonsil and skin mast cells were investigated in the current study. We reported that histamine was able to activate gut mast cells, which presents a self-amplification mechanism of mast cell degranulation[14]. Since human mast cells from different anatomical sources may respond to a stimulus to different extents, which has long been known as mast cell heterogeneity[15], the effect of histamine on tryptase release from human tonsil and skin mast cells was also examined in this study.
Materials and methods
Dispersion of mast cells Human tonsil and skin tissue were obtained at tonsillectomy and circumcision, from the Pathology Department of Shantou University Medical College (Shantou, China). Informed consent from the patients and agreement of the Ethical Committee of the college were obtained. Only macroscopically normal tissues were used for the study. The mast cell dispersion procedures employed were similar to that described previously[16]. Briefly, finely chopped tissue was incubated with 1.5 g/L collagenase (Sigma, USA) and 0.75 g/L hyaluronidase (Sigma) in minimum essential medium (MEM, Gibco, Invitrogen Corpora-tion, USA) containing 25 mmol/L N-2-hydroxylethyl-pipera-zine-N'-2-ethane sulphonic acid (HEPES) and 2% foetal calf serum (FCS, 1 g tonsil/10 mL buffer and 1 g skin/15 mL buffer) at 37 °C for 60–70 min. Dispersed cells were separated from undigested tissue by filtration through nylon gauze (pore size 100 µm diameter), washed and maintained in MEM (containing 10% FCS, 200 kU/L benzylpenicillin, and 200 mg/L streptomycin) on a roller overnight at room temperature. Mast cell purity, as determined by light microscopy after staining by alcine blue, ranged from 0.5% to 1.1% for tonsil cells and 3.5% to 5.8% for skin cells.
Mast cell challenge Dispersed cells were resuspended in HEPES buffered salt solution (HBSS, pH 7.4) with CaCl2 and MgCl2 (complete HBSS). Aliquots of 100 µL containing 4×103–6×103 mast cells were added a 50-µL of tc-LIGRLO (Meilian, GuZhen Town, China), tc-OLRGIL (Meilian), SLIGKV (Meilian), VKGILS (Meilian), trypsin (Sigma), tryptase (self-prepared[17]), histamine (Sigma), anti-IgE antibody (Serotec, Oxford, UK), calcium ionophore (CI) (Sigma), SBTI (Sigma), or α1-AT (Sigma), and incubated at 37 °C for 15 min. The reaction was terminated by addition of 150 µL ice cold incomplete HBSS and tubes were centrifuged immediately (500×g, 10 min, 4 °C). All experiments were performed in duplicate. For measuring of total histamine or tryptase concentrations, four tubes were either boiled for 6 min or the freeze-thaw cycle was repeated five times. Supernatants were stored at -20 °C until tryptase and histamine concentrations were determined (in duplicate for each tube).
Inhibition of release of tryptase or histamine When added, SBTI or α1-AT were incubated with trypsin for 20 min on ice before adding to cells. Data were expressed as the percentage inhibition of tryptase or histamine release, taking into account histamine or tryptase release in presence and absence of the inhibitor. When the experiments with pertussis toxin were performed, cells were incubated with 1.0 mg/L pertussis toxin at 37 °C for 4 h, and then washed with HBSS before stimulus being added. When the experiments with metabolic inhibitors were performed, cells were incubated with 2-deoxy-D-glucose (10 mmol/L, Sigma) and antimycin A (1 µmol/L, Sigma) at 37 °C for 40 min before being challenged with stimulus.
Tryptase and histamine measurement Tryptase concentrations were measured with a sandwich ELISA procedure with a specific polyclonal antibody against human tryptase as the capture antibody and AA5 a monoclonal antibody specific for human tryptase (both donated by Dr Andrew FW, University of Southampton, UK) as the detecting antibody[18]. Histamine concentrations were determined using a glass fibre-based fluorometric assay[16].
Statistical analysis Statistical analysis were performed by using SPSS software. Data were expressed as mean±SD. Where analysis of variance indicated significant differences between groups with ANOVA, for the preplanned comparisons of interest, Student’s t-test was applied. P<0.05 was taken as a statistically significant difference.
Results
Effect of PAR-2 agonists and their reverse peptides on tryptase and histamine release from tonsil and skin mast cells PAR-2 agonist peptide SLIGKV at the concentrations of 1, 10, and 100 µmol·L-1 was able to induce a dose-dependent release of histamine (ranging from 8.9% to 13.8% net histamine release), but not tryptase (data not shown) from skin mast cells. However, SLIGKV at 300 µmol·L-1 failed to induce histamine release from skin mast cells. In the same experiments, a reverse peptide of SLIGKV, VKGILS had little effect on histamine release. In contrast to skin mast cells, tonsil mast cells released more tryptase, but not histamine (data not shown) in response to SLIGKV. In the same experiments, VKGILS induced significantly less tryptase release from tonsil cells than SLIGKV did. Another PAR-2 agonist peptide tc-LIGRLO was able to induce significant release of histamine from skin mast cells and release of tryptase from tonsil mast cells, but the extent of histamine and tryptase release induced by tc-LIGRLO appeared less than that induced by its reverse peptide tc-OLRGIL (Table 1).
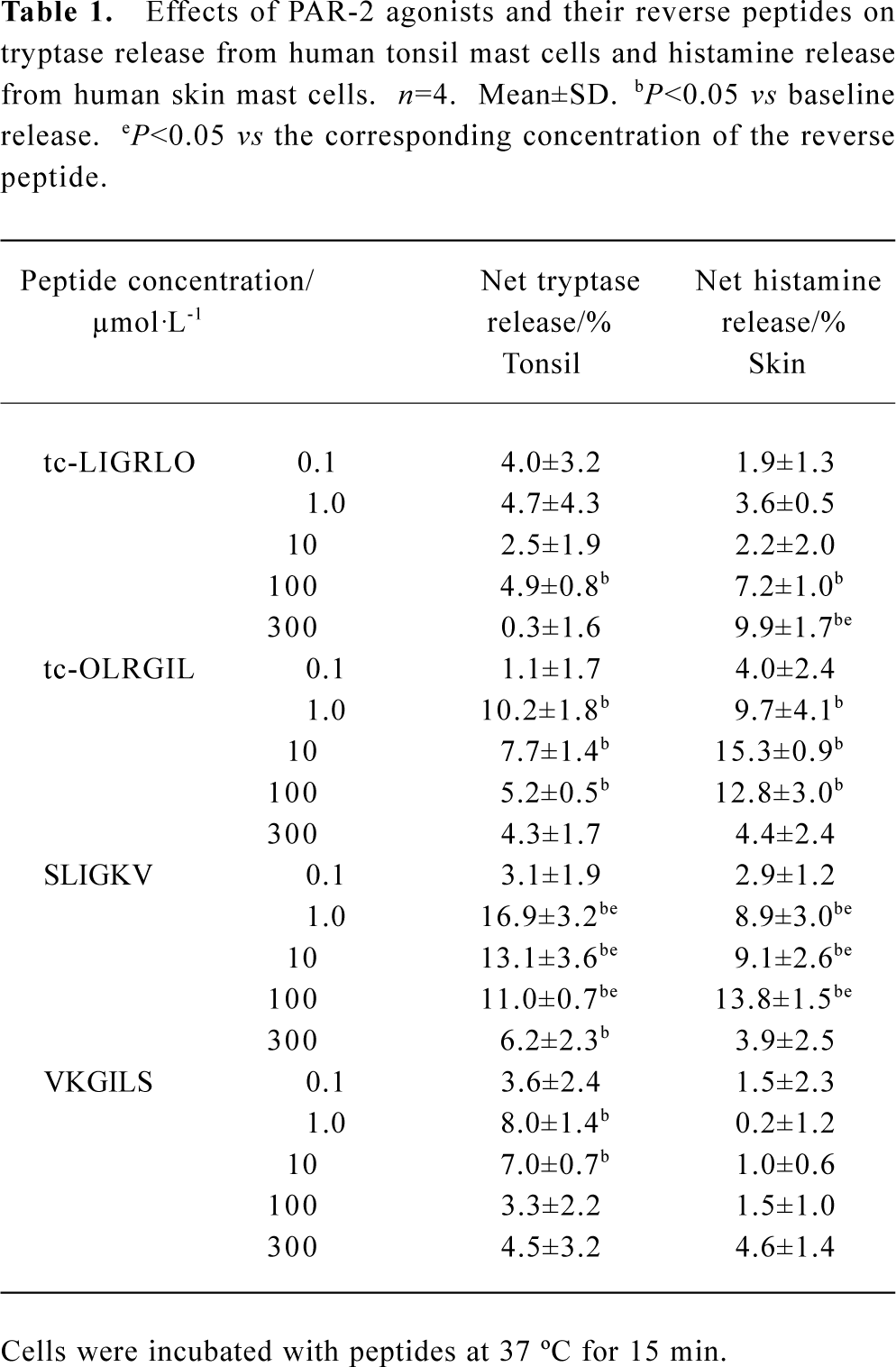
Full table
Effects of trypsin, anti-IgE, CI, and histamine on tryptase release from tonsil and skin mast cells Trypsin was able to induce a “bell” shape increase in tryptase release from tonsil mast cells. The maximum of net tryptase release was 19.4% induced by 1 mg/L trypsin (Figure 1). With the same experimental procedure, trypsin failed to induce significant tryptase release from skin mast cells (Figure 2). Anti-IgE was able to stimulate tryptase release from both tonsil and skin mast cells (Figure 1, 2). The maximum release of tryptase from tonsil cells was 17.7% induced by 10 mg/L anti-IgE. CI at the concentrations of 0.1, 0.3, and 1 mg/L was able to provoke a dose-dependent release of tryptase from tonsil mast cells (Figure 1). The maximum release of tryptase from tonsil cells was 22.8% induced by 1 mg/L CI (Figure 1). CI at the concentration of 1 mg/L was also able to induce significant tryptase release from skin mast cells (Figure 2).
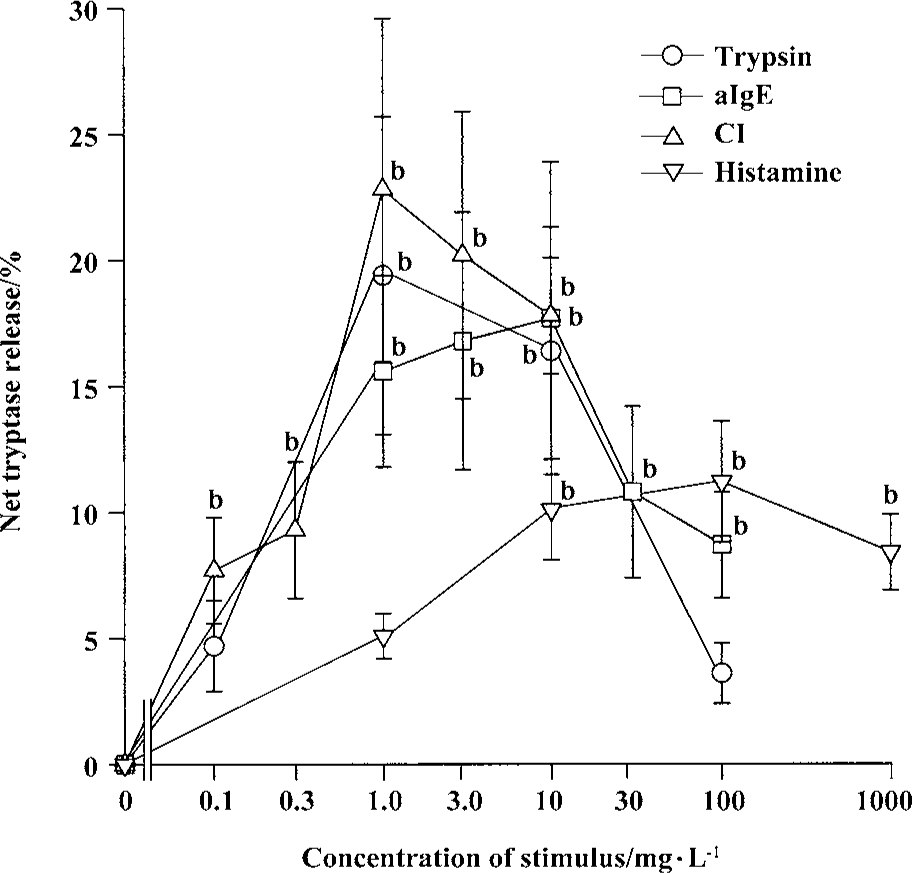
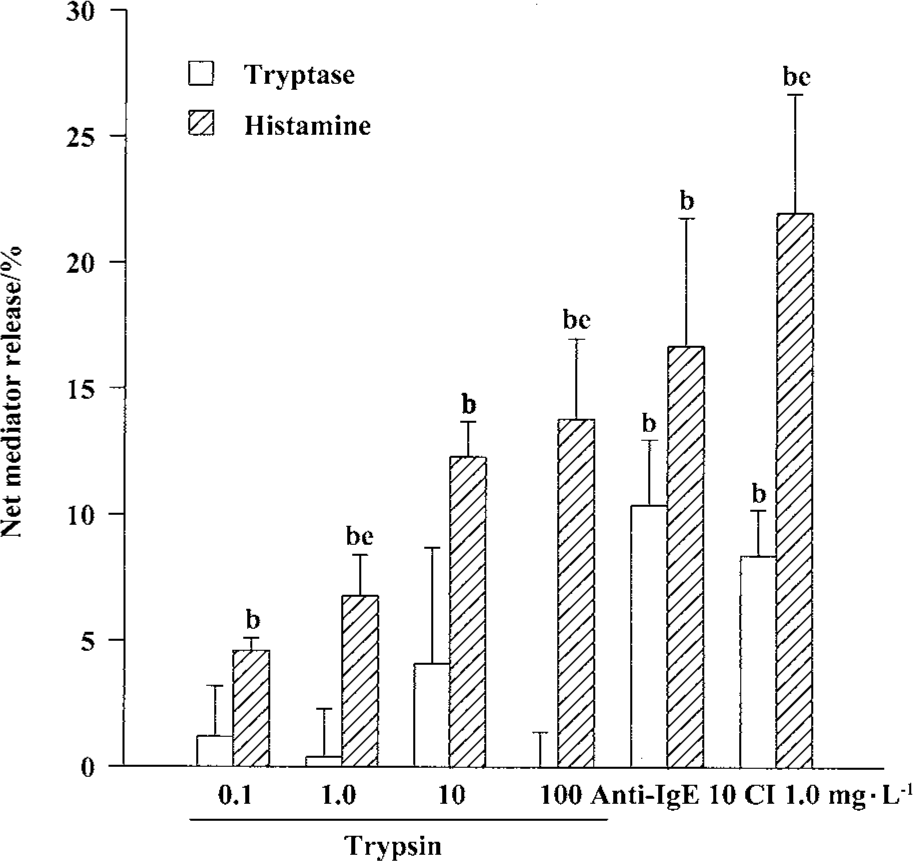
Effects of trypsin, anti-IgE, CI, and tryptase on histamine release from tonsil and skin mast cells Trypsin at the concentrations of 0.1, 1.0, 10, and 100 mg/L was able to induce a dose-dependent release of histamine from skin mast cells (Figure 2). The maximum of histamine release was 13.9% induced by100 mg/L trypsin. Trypsin at the concentrations of 10 and 100 mg/L was also able to induce significant histamine release from tonsil mast cells (Figure 3). Similarly, anti-IgE and CI were able to induce a dose-dependent release of histamine from tonsil mast cells. Up to12.8% release of histamine from tonsil mast cells was observed when cells were incubated with tryptase 10 mg/L (Figure 3).
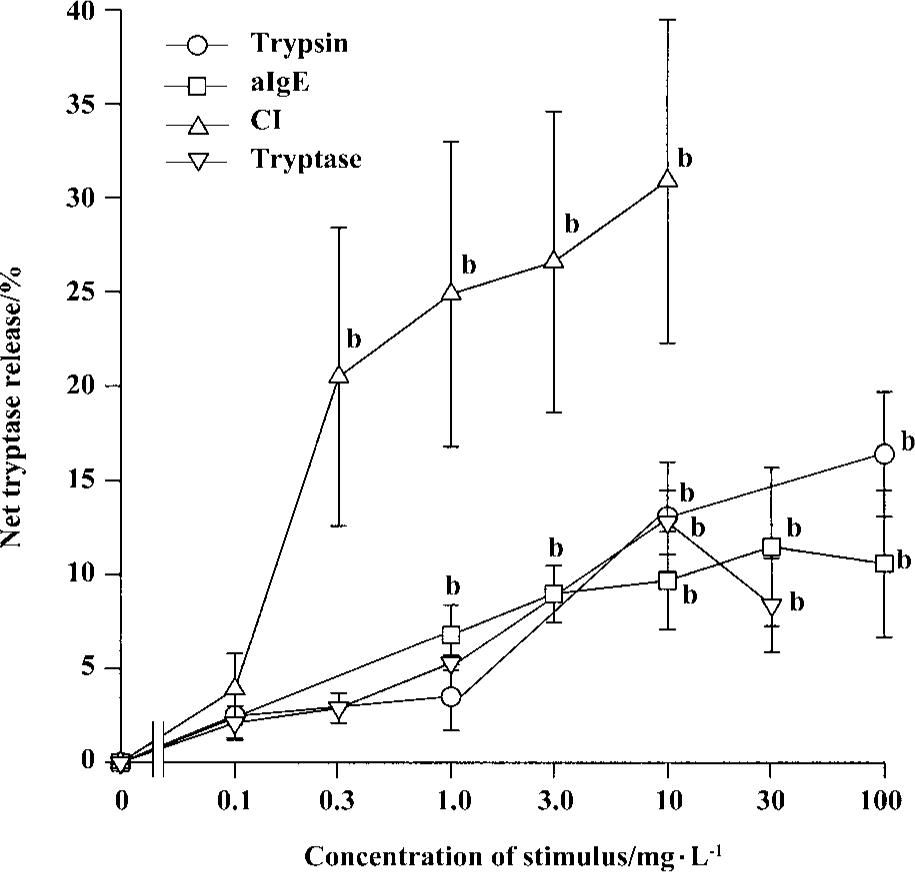
Time course for tryptase and histamine release from tonsil mast cells Time course study revealed that both tryptase and histamine release induced by anti-IgE, histamine, and CI from tonsil mast cells initiated within 10 s when cells were incubated with stimulus. Up to 45% and 31% of the maximum tryptase and histamine release were observed 10 s after cells were incubated with stimulus. The peak tryptase release from tonsil cells occurred at 4 min for CI and histamine, and 6 min for anti-IgE following incubation (Figure 4). In comparison, the peak of histamine release induced by anti-IgE or CI occurred at 5 min and 6 min, respectively (Figure 4). Similarly, tc-LIGRLO, trypsin, and CI-induced histamine release from skin mast cells all started within 10 s of stimulation. But the peak histamine release occurred at 4 min for CI and 6 min for tc-LIGRLO and trypsin (Figure 5).
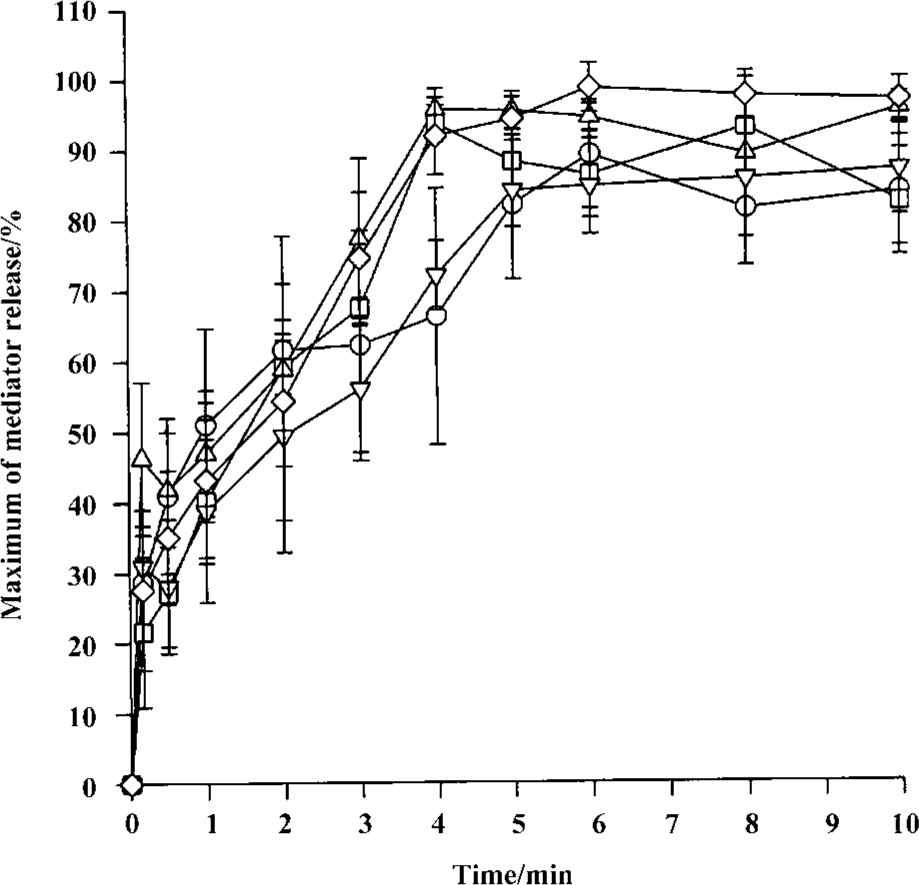
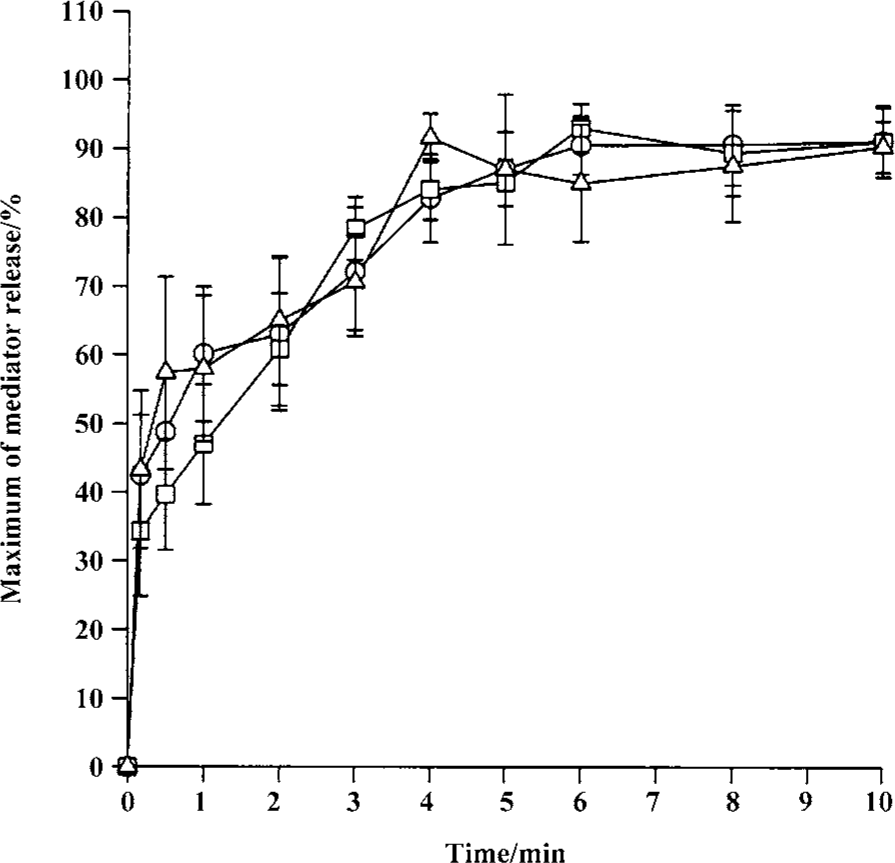
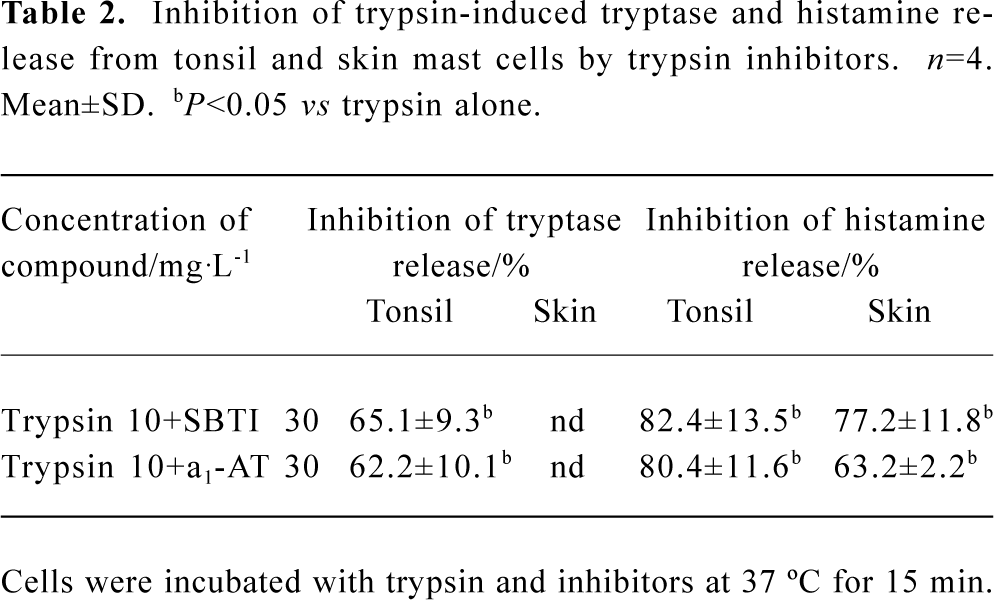
Inhibition of trypsin-induced tryptase and histamine release by trypsin inhibitors The trypsin-induced tryptase release from tonsil mast cells was inhibited by approximately 65.1% and 62.2% by SBTI or α1-AT, respectively (Table 2). Similarly, the trypsin-induced histamine release from tonsil mast cells was inhibited by approximately 82.4% and 80.4% by SBTI or α1-AT, respectively (Table 2). SBTI or α1-AT were also able to inhibit trypsin-induced histamine release from skin mast cells by 77.2% and 63.2%, respectively (Table 2).
Full table
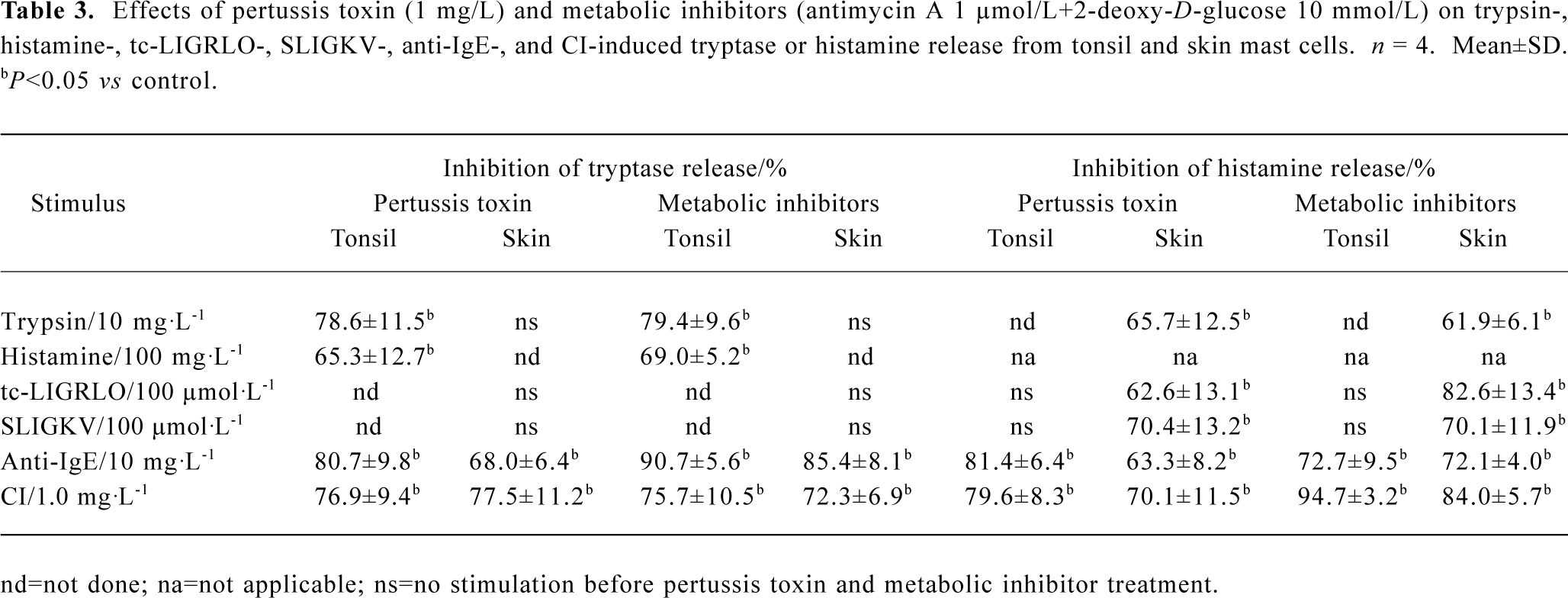
Inhibition of tryptase and histamine release by pertussis toxin and metabolic inhibitors Pretreatment of cells with metabolic inhibitors (10 mmol/L of 2-deoxy-D-glucose and 1 µmol/L of antimycin A) reduced trypsin-induced tryptase release from tonsil cells and histamine release from skin cells by 79.4% and 61.9%, respectively. Metabolic inhibitors were also able to inhibit histamine-induced tryptase release from tonsil mast cells, and tc-LIGRLO and SLIGKV-induced histamine release from skin mast cells. Pretreatment of cells with 1 mg/L pertussis toxin diminished both trypsin and histamine-induced tryptase release from tonsil mast cells, and trypsin, tc-LIGRLO, and SLIGKV-provoked histamine release from skin mast cells. Similarly, metabolic inhibitors and pertussis toxins were able to inhibit both anti-IgE and CI-induced tryptase and histamine release from both tonsil and skin mast cells (Table 3).
Full table
Discussion
We have reported the in vitro tryptase release properties of human tonsil and skin mast cells. This was particularly important when tryptase and histamine, the two major mast cell mediators were investigated in parallel in the same experiments, which not only proved that the quantities of the two mediators released from mast cells were in an inconstant ratio, but also revealed that the pace of release was different between histamine and tryptase upon mast cell degranulation. These phenomena were similar to our previous findings with human colon mast cells[12].
Trypsin at 1 mg/L was able to stimulate the maximum tryptase release (19.4%), but not significant histamine release from tonsil mast cells. In contrast, trypsin at 100 mg/L was able to stimulate 16.4% histamine release, but not significant tryptase release from tonsil mast cells. These are surprising observations, which suggests strongly that tryptase and histamine release from tonsil mast cells induced by trypsin may be involved in different mechanisms. Since anti-IgE and CI also showed “bell” shape tryptase release and dose-dependent histamine release patterns in the same experimental system, these observations may reflect a common phenomenon of tonsil mast cell degranulation. It is most likely that tryptase release pathway of tonsil mast cells has the ability to prevent itself from strong stimulation. However, more experiments are needed to prove this. In contrast to mast cells from tonsil, mast cells from skin released only histamine, but not tryptase in response to trypsin, which indicated further that tryptase and histamine release from human mast cells induced by trypsin was through different mechanisms. The difference in the ability of trypsin inhibitors to inhibit trypsin induced histamine release (up to 82.4%) and tryptase release (up to 65%) may also suggest the different mechanisms involved in tryptase and histamine release from tonsil mast cells.
Moreover, PAR-2 agonist peptides SLIGKV and tc-LIGRLO showed different effects on tryptase and histamine release from mast cells. As for trypsin, SLIGKV was also able to stimulate histamine release, but not tryptase release from skin mast cells. The extent of histamine release induced by SLIGKV was similar to that induced by trypsin, indicating that the actions of trypsin on skin mast cells may be through PAR-2. Because VKGILS (a reversed peptide of SLIGKV) had little effect on tryptase and histamine release from skin mast cells, the action of SLIGKV on skin mast cells was a specific one. Different from skin mast cells, tonsil mast cells released tryptase but not histamine in response to SLIGKV. This suggested that trypsin induced histamine release from tonsil mast cells might not be through a PAR-2 related mechanism. It suggested also that release of tryptase and histamine from tonsil mast cells upon stimulation might not occur simultaneously. Another PAR-2 agonist peptide tc-LIGRLO had a similar effect to SLIGKV on skin mast cells, confirming further the functional expression of PAR-2 on these cells. Interestingly, tc-OLRGIL a reversed peptide of tc-LIGRLO appeared a potent stimulus of histamine release from skin mast cells, and a secretagogue of tryptase release from tonsil mast cells. The physiological or pathophysiological meaning of the action of tc-OLRGIL on mast cells requires further investigation to be understood.
Consistent with our previous findings[14,15], histamine exhibited its ability to stimulate tryptase release from tonsil mast cells, and tryptase showed its ability to provoke histamine release from mast cells. These implicated that there were at least two self-amplification mechanisms of mast cell degranulation in the human tonsil; the histamine associated mechanism and the tryptase associated mechanism.
Approximately 30% tryptase and histamine release occurred within 10 s of IgE-dependent stimulation, suggesting that tonsil mast cells were able to quickly respond to allergen challenge, but to reach their full capacity (maximum histamine or tryptase release) a minimum of 5 min was required. The time courses for CI were similar to those for anti-IgE, except for a minimum 4 min being required to reach the full tryptase and histamine release capacity. The reasons for the relatively slow release of mediators from human mast cells remain unclear. Approximately 45% tryptase release induced by histamine was completed within the first 10 s of stimulation, indicating that the process may be different from the one induced by anti-IgE or CI. The time courses for histamine release from skin mast cells induced by CI and tc-LIGRLO were quite similar, indicating tc-LIGRLO may act like CI on induction of mast cell degranulation.
Pretreatment of cells with metabolic inhibitors abolished the actions of anti-IgE and CI on mast cells, indicating that tryptase and histamine release induced by them was a non-cytotoxic process, and was dependent on cell energy supply. The inhibition of tryptase and histamine release by pertussis toxin suggested that the release process was associated with activation of G-protein coupled receptors.
In conclusion, it was found that tryptase release properties of human tonsil and skin mast cells were similar to the histamine release properties of these cells in response to anti-IgE and CI. In contrast, tryptase release properties were quite different from histamine release properties in response to PAR-2 agonists including trypsin, tc-LIGRLO, and SLIGKV, which uncovered a novel type of mast cell heterogeneity in response to different stimulation. The activation of mast cells by PAR-2 agonists may indicate a self-amplification mechanism of mast cell degranulation in humans.
References
- He S. The key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol 2004;10:309-18.
- Walls AF, He S, Buckley MG, McEuen AR. Roles of the mast cell and basophil in asthma. Clin Exp Allergy Rev 2001;1:68-72.
- He S, Chen HQ, Zheng J. Inhibition of tryptase and chymase induced nucleated cell infiltration by proteinase inhibitors. Acta Pharmacol Sin 2004;25:1677-84.
- Schwartz LB. Preformed mediators of human mast cells and basophils. In: Holgate ST, editor. Mast cells, mediators and disease. Dordrect: Kluwer Academic Publishers; 1988. p 129–47.
- McEuen AR, He S, Brander ML, Walls AF. Guinea pig lung tryptase: Localisation to mast cells and characterisation of the partially purified enzyme. Biochem Pharmacol 1996;52:331-40.
- Okayama Y, Church MK. Comparison of the modulatory effect of ketotifen, sodium cromoglycate, procaterol and salbutamol in human skin, lung and tonsil mast cells. Int Arch Allergy Appl Immunol 1992;97:216-25.
- Okayama Y, Benyon RC, Lowman MA, Church MK. In vitro effects of H1-antihistamine and PGD2 release from mast cells of human lung, tonsil, and skin. Allergy 1994;49:246-53.
- Butchers PR, Vardey CJ, Johnson M. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br J Pharmacol 1991;104:67-26.
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem 1997;272:4043-9.
- D’Andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech Histochem 2000;75:85-90.
- Stenton GR, Nohara O, Dery RE, Vliagoftis H, Gilchrist M, Johri A, et al. Proteinase-activated receptor (PAR)-1 and -2 agonists induce mediator release from mast cells by pathways distinct from PAR-1 and PAR-2. J Pharmacol Exp Ther 2002;302:466-74.
- He S, He Y, Xie H. Activation of human colon mast cells through proteinase activated receptor-2 (PAR-2). World J Gastroenterol 2004;10:327-31.
- Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol 1997;75:832-41.
- He S, Xie H. Modulation of tryptase secretion from human colon mast cells by histamine. World J Gastroenterol 2004;10:323-26.
- He S, Gaça MDA, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther 1998;286:289-97.
- He S, Xie H, Zhang XJ, Wang XJ. Inhibition of histamine release from human mast cells by natural chymase inhibitors. Acta Pharmacol Sin 2004;25:822-6.
- He S, Chen P, Chen HQ. Modulation of enzymatic activity of human mast cell tryptase and chymase by proteinase inhibitors. Acta Pharmacol Sin 2003;24:923-9.
- Buckley MG, Walters C, Brander M, Wong WM, Cawley MI, Ren S, et al. Mast cell activation in arthritis: detection of α- and β-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci 1997;93:363-70.
