Similar effects on rat renal mesangial cells by expressing different fragments of adrenomedullin gene in vitro1
Introduction
Adrenomedullin (AM) was discovered in tissue extracts of human pheochromocytoma by Kitamura et al in 1993[1]. Subsequently identified as a potent vasodilator, AM was detected to be expressed in a wide range of tissues, such as the adrenal gland, kidney, heart, lung, spleen, and brain[2]. AM has since been suggested to play important roles in a great number of disease areas, “ranging widely from heart failure through to oncology”, especially in cardiovascular and renal diseases[3,4]. Previous studies revealed that plasma AM levels were increased in hypertension and renal failure[5,6]. Researches have also shown that AM can improve cardiac function, renal function, and survival rate in hypertensive rat models[7,8].
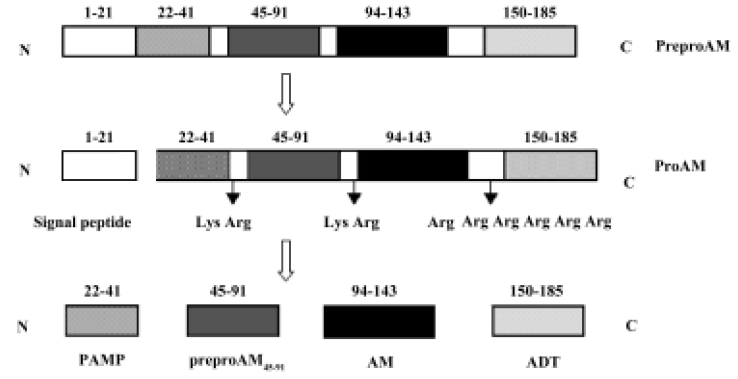
The gene encoding preproadrenomedullin (preproAM), a precursor molecule of AM, is termed as the AM gene. Sequence analysis of cloned rat AM showed that this precursor consisted of 185 amino acids, including a signal peptide[9]. After synthesis, the signal peptide is cleaved between Thr21 and Ala22. The remaining fragment of preproAM, which is termed as proadrenomedullin (proAM), is cleaved specifically by endopeptidase at Lys43–Arg44, Lys92–Arg93 and Arg145–Arg149, resulting in the production of four proAM-derived peptides: PAMP (proadrenomedullin N-terminal 20 peptide, preproAM22–41), preproAM45–91, AM (preproAM94–143) and ADT (adrenotensin or preproAM150–185)[9,10] (Figure 1).
Finding out the appropriate expression pattern is one of the major topics in gene delivery. It is well known that peptides derived from a common polypeptide precursor may have different, even opposite biological properties. Previous studies suggest that ADT may play a role that is opposite in effect to AM[11,12]. The present study was carried out in order to explore the different effects of expressing full length preproAM and a fragment of preproAM without ADT on renal mesangial cells (RMC).
Materials and methods
Materials pEGFP-N3 vector was obtained from BD Biosciences Clontech (Bedford, MA,USA). Top10 Escheri-chia coli was obtained from Invitrogen (Carlsbad, CA, USA) and primary rat RMC was a gift from the Department of Pathology, Shanghai Medical College (Shanghai, China).
Reagents Trizol, AMV reverse transcriptase, Taq DNA polymerase, Silver Beads DNA Gel Extraction Kit, kits for total RNA extraction, endonucleases EcoRI and BamHI, and T4 DNA ligase were purchased from Sangon (Shanghai, China). LipofectamineTM 2000 was from Gibco (Grand Island, NY, USA), and MTT, dimethyl sulfoxide (Me2SO) and Dulbecco’s Modified Eagle’s Medium (DMEM)/F-12 were from Sigma (St Louis, MO, USA).
Construction and confirmation of expression of vectors pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 Total RNA was isolated from rat kidney tissue samples using Trizol reagent. The full coding region of the AM gene was generated by reverse transcription-polymerase chain reaction (RT-PCR) using the following oligonucleotide primers: AM-1, 5'-TAC TGA ATT CGC CAC CAT GAA GC-3'; and AM-2, 5'-TTG CGG ATC CTA ACC TAG AGA C-3'. After an initial denaturation step at 94 °C for 3 min, PCR were carried out for 30 cycles at 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, and then 72 °C for 10 min. The PCR products and pEGFP-N3 were digested with restriction endonucleases EcoRI and BamHI (recognition sites in bold) and the purified digestion products were subcloned into the EcoRI and BamHI sites of pEGFP-N3 with T4 DNA ligase. The recombinant plasmids were then transformed into competent Top10 E coli and amplified in the hosts. DNA sequencing was carried out to identify purified recombinant vectors. The recombinant vector carrying the full coding region of the rat AM gene was named pEGFP-N3-AM1-2. Using the same method (with oligonucleotide primers: AM-1, 5'-TAC TGA ATT CGC CAC CAT GAA GC-3'; and AM-3, 5'-TTG CGG ATC CAT AGC CTT GAG-3'), we developed another recombinant pEGFP-N3 vector (pEGFP-N3-AM1-3) with a fragment encoding a region of the rat AM gene in which the sequence encoding ADT was absent.
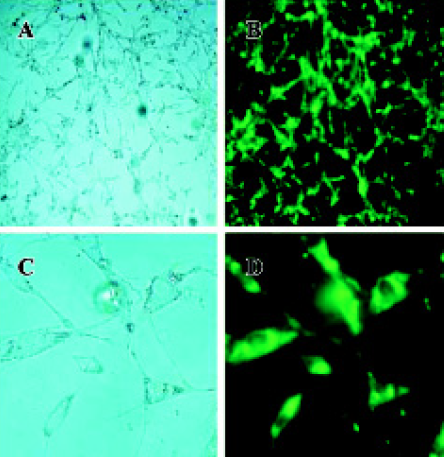
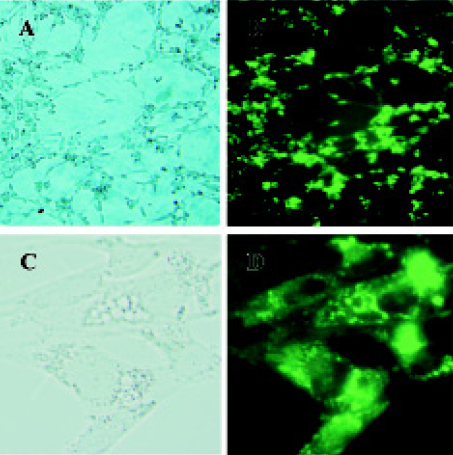
Cell culture and transfection Primary rat mesangial cells were cultured in DMEM/F-12 containing 10% heat-inactivated fetal calf serum (FCS) and incubated in a humidified 5% CO2 incubator at 37 °C. Cells were growth-arrested in DMEM containing 1.0% FCS for 24 h prior to transfection. pEGFP-N3-AM1-2 or pEGFP-N3-AM1-3 was transfected into cultured RMC facilitated by cationic liposomes Lipofecta-mineTM 2000. At 24 h after transfection, phase contrast and fluorescence microscopy were carried out using a BX61olym-pus microscope (Melville, NY, USA)
Detection of cell proliferation by MTT assay Cellular proliferation was determined in triplicate with a colorimetric non-radioactive MTT proliferation assay. RMC were plated at 3×103 cells/well into 96-well plates and cultured in DMEM/F-12 medium supplemented with 10% FCS at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. The cultures were replaced with medium containing 1.0% FCS for another 24 h, and the cells were then transfected with pEGFP-N3-AM1-2 or pEGFP-N3-AM1-3 for 48 h. The effects of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 on the proliferation of RMC were measured by MTT assay. Briefly, a 20 µL aliquot of 5 mg/mL MTT solution was added to each well. After 4 h of incubation, the absorbance of each well was measured at 490 nm.
Reverse transcription-polymerase chain reaction analysis of transforming growth factor-β1 gene expres-sion Expression of the transforming growth factor-β1 (TGF-β1) gene was analyzed by semiquantitative PCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Total RNA was isolated from cultured RMC following the protocol of RNA extraction kits. The amount of RNA isolated was determined by measuring the specific absorption at 260 nm. The integrity of the RNA isolated was confirmed by agarose gel electrophoresis, run under denaturing conditions. cDNA was synthesised in 40 µL reaction mixtures using 1 µg of total RNA; 2 µL of the cDNA solution was used for PCR amplification. The primers for the TGF-β1 and GAPDH genes were as follows: TGF-β1 sense, 5'-AAG TGG ATC CAC GAG CCC AA-3'; TGF-β1 antisense, 5'-GTC GCA CTT GCA GGA GCG CA-3'; GAPDH sense, 5'-ACC ACA GTC CAT GCC ATC AC-3'; GAPDH antisense, 5'-CCA CCA CCC TGT CAT GCC ATC AC-3'. After an initial denaturation step at 94 °C for 3 min, PCR was carried out for 30 cycles and 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, and then at 72 °C for 10 min.
Statistical analysis Statistical analysis was carried out using the statistical program SPSS 11.0 (Chicago, Illinois, USA). The data were expressed as mean±SD and the one-way analysis of variance (ANOVA) t-test was used for statistical analysis. P<0.05 was accepted as statistically significant.
Results
Construction and identification of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 The full coding region of the rat AM gene and the fragment of coding region without ADT were subcloned separately into pEGFP-N3, producing pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3, respectively. The construction of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 was confirmed by DNA sequencing.
Expression of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 in renal mesangial cells Cultured RMC were transfected with pEGFP-N3-AM1-2 or pEGFP-N3-AM1-3. After 24 h, green fluorescence was detected using a fluorescence microscope. Both pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 were expressed in RMC as bright green fluorescence was observed, indicating that the in-frame GFP-tagged AM sequences were expressed and translated successfully. The expression of GFP was observed in above 95% transfected RMC. No green fluorescence was detected in untransfected RMC (Figures 2,3,4).
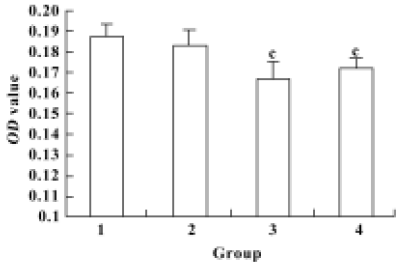
Proliferation of renal mesangial cells in response to pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 To determine the biological activities of the recombinant vectors, proliferation of RMC was analyzed using the MTT assay 48 h after pEGFP-N3-AM1-2 or pEGFP-N3-AM1-3 delivery. Significant changes in optical density values at 24 h after transfection was observed with recombinant plasmids compared with the controls, indicating that pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 elicited a decrease in RMC proliferation (P<0.01) (Figure 5).
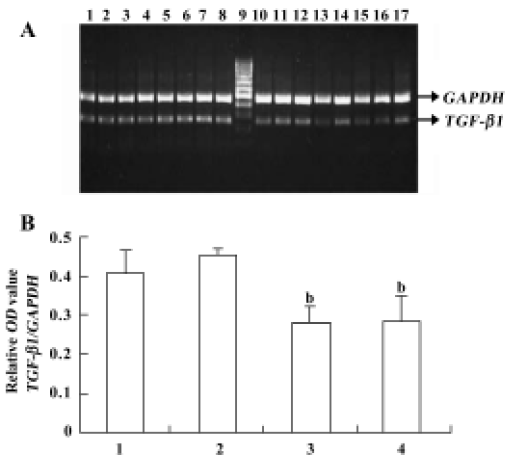
Analysis of the effects of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 on TGF-β1 gene expression Along with the control experiments, which indicated that product accumulation did not reach a plateau at 30 cycles under the assay conditions used, our results for semiquantitative PCR indicate that, compared with the control group, transfection of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 suppressed TGF-β1 mRNA expression in RMC (Figure 6).
Discussion
As an endogenous vasodilator and natriuretic peptide, AM is thought to have great potential in the treatment of cardiovascular and renal diseases[13,14]. However, there are still some impediments to understand AM’s biological functions and the promising clinical uses of AM. One of the obstacles is the short half-life of AM. The plasma half-life of AM is estimated to be about only 22 min[15]. Gene transfer is a novel means of providing sustained and localized delivery of the required therapeutic protein. In order to achieve long-term expression of AM, AM gene delivery has been used in recent years. The studies show that AM gene delivery has multiple functions in protection against hypertension, renal damage, cardiac fibrosis and cardiac hypertrophy[16–20].
Currently, both non-viral and viral vectors are used to express the heterogeneous genes, each of them with their own advantages and disadvantages. The advantages of non-viral vectors are: (i) non-vial vectors do not integrate the heterogeneous genes into recipient-cell genome, therefore it is considered safe for researchers and will have higher clinical acceptability; (ii) there are minimal or no immune effects, and none of the safety concerns regarding infection is found with viral vectors[21]; (iii) manipulate and delivery of plasmids into cells is much easier; and (iv) although plasmid-delivered DNA does not integrate into the recipient-cell genome, delivery of plasmids in vivo can maintain very prolonged effects[22]. In the present study, we chose plasmid pEGFP-N3 as the vector.
In our experiment, for the first time, the recombinant vector pEGFP-N3-AM1-3, which carries the DNA fragment encoding the AM protein without the ADT sequence, was designed and constructed successfully. It is well known that peptides derived from a common polypeptide precursor may have different, even opposite biological properties. As prior studies have demonstrated, ADT has some quite opposite vascular effects to those of AM[11,12]. We therefore hypothesized that the absence of ADT might have more effective effects in protecting heart and kidney.
The pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 plasmids were constructed successfully. As the fluorescent images showed, both of these vectors were expressed efficiently in RMC. Our data analysis showed that either pEGFP-N3-AM1-2 or pEGFP-N3-AM1-3 delivery inhibited the proliferation of RMC (P<0.01) and decreased the mRNA transcription of TGF-β1 in RMC (P<0.05). However, no significant difference was observed between the effects of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3.
In kidney, mesangial cells have a central function in maintaining structural and functional integrity of the glomerulus, including “structural support of the capillary tuft, modulation of glomerular hemodynamics, and a phagocytic function allowing removal of macromolecules and immune complexes”, and the proliferation of mesangial cells is a pivotal feature of glomerular disease[23]. Our results are in agreement with prior studies showing that AM can suppress RMC proliferation[24–26], and this may be one of the mechanisms by which AM gene delivery protects renal function. So far, no data about the effects of ADT on the proliferation of mesangial cells have been reported.
Transforming growth factor-β1 is a multifunctional peptide that regulates manifold cellular functions including cell proliferation, differentiation and apoptosis[27]. As a potent inducer of extracellular matrix synthesis leading to progressive glomerular fibrosis, TGF-β1 is also thought to be the major pathogenic factor in the development of renal fibrotic disorders[28]. A previous study showed that chronic AM infusion significantly reduced the TGF-β/GAPDH mRNA levels in the renal cortex[7]. In the present study, we demonstrated that either pEGFP-N3-AM1-2 or pEGFP-N3-AM1-3 delivery could decrease the mRNA transcription level of TGF-β1 in RMC (P<0.05). This may contribute to the renal protective effects of AM gene delivery; the function of ADT in the regulation of TGF-β1 mRNA expression requires further study.
In the present experiment, a very interesting phenomenon was observed in that there was no difference between pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 in their effects on the proliferation of RMC and the mRNA transcription level of TGF-β1 in cultured RMC. Although ADT plays some opposite vascular effects to AM, previous studies indicate that there are complicated mutual regulation interactions between ADT and AM. In incubated blood vessels, AM could inhibit the synthesis and release of ADT, whereas ADT could enhance the synthesis and release of AM[29]. Therefore, in order to understand the mechanism of why pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 had similar effects on cultured RMC, further investigations are indispensable.
In conclusion, recombinant vectors pEGFP-N3-AM1-2, expressing preproAM, and pEGFP-N3-AM1-3, expressing a fragment of preproAM without ADT, were constructed successfully. The biological activity studies of pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 on cultured RMC showed that expressing the fragment of preproAM without ADT and the full-length preproAM had similar inhibitory effects on cell proliferation and TGF-β1 gene transcription. As for the mechanism of why pEGFP-N3-AM1-2 and pEGFP-N3-AM1-3 had similar effects on cultured RMC, and what may be the effects of the 2 vectors on the kidney in vivo, further investigations are required.
References
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. AM: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993;192:553-60.
- Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T. Distribution and characterization of immunoreactive AM in human tissue and plasma. FEBS Lett 1994;338:6-10.
- Beltowski J, Jamroz A. Adrenomedullin–what do we know 10 years since its discovery? Pol J Pharmacol 2004;56:5-27.
- Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. The clinical relevance of adrenomedullin: a promising profile? Pharmacol Ther 2004;103:179-201.
- Ishimitsu T, Nishikimi T, Saito Y, Kitamura K, Eto T, Kangawa K, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest 1994;94:2158-61.
- Kato J, Kobayashi K, Etoh T, Tanaka M, Kitamura K, Imamura T, et al. Plasma adrenomedullin concentration in patients with heart failure. J Clin Endocrinol Metab 1996;81:180-3.
- Mori Y, Nishikimi T, Kobayashi N, Ono H, Kangawa K, Matsuoka H. Long-term adrenomedullin infusion improves survival in malignant hypertensive rats. Hypertension 2002;40:107-13.
- Chao J, Chao L. The role of adrenomedullin in cardiovascular and renal function. Drug News Perspect 2002;15:511-8.
- Sakata J, Shimokubo T, Kitamura K, Nakamura S, Kangawa K, Matsuo H, et al. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun 1993;195:921-7.
- Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 2000;21:138-67.
- Gumusel B, Chang JK, Hyman A, Lippton H. Adrenotensin: an ADM gene product with the opposite effects of ADM. Life Sci 1995;57:PL87-90.
- Gumusel B, Chang JK, Hao Q, Hyman A, Lippton H. Adrenotensin: an adrenomedullin gene product contracts pulmonary blood vessels. Peptides 1996;17:461-5.
- Nakamura R, Kato J, Kitamura K, Imamura T, Eto T. Potential of adrenomedullin as a therapeutic tool for left ventricular remodeling after myocardial infarction. Nippon Rinsho 2004;62:302-6.
- Savoia C, Schiffrin EL. Significance of recently identified peptides in hypertension: endothelin, natriuretic peptides, adreno-medullin, leptin. Med Clin North Am 2004;88:39-62.
- Meeran K, O’Shea D, Upton PD, Small CJ, Ghatei MA, Byfield PH, et al. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab 1997;82:95-100.
- Chao J, Jin L, Lin KF, Chao L. Adrenomedullin gene delivery reduces blood pressure in spontaneously hypertensive rats. Hypertens Res 1997;20:269-77. [published erratum appears in Hypertens Res 2001; 24: 611].
- Dobrzynski E, Montanari D, Agata J, Zhu J, Chao J, Chao L. Adrenomedullin improves cardiac function and prevents renal damage in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 2002;283:E1291-8.
- Wang C, Dobrzynski E, Chao J, Chao L. Adrenomedullin gene delivery attenuates renal damage and cardiac hypertrophy in Goldblatt hypertensive rats. Am J Physiol Renal Physiol 2001;280:F964-71.
- Zhang JJ, Yoshida H, Chao L, Chao J. Human adrenomedullin gene delivery protects against cardiac hypertrophy, fibrosis, and renal damage in hypertensive dahl salt-sensitive rats. Hum Gene Ther 2000;11:1817-27.
- Dobrzynski E, Wang C, Chao J, Chao L. Adrenomedullin gene delivery attenuates hypertension, cardiac remodeling, and renal injury in deoxycorticosterone acetate-salt hypertensive rats. Hypertension 2000;36:995-1001.
- Pachori AS, Huentelman MJ, Francis SC, Gelband CH, Katovich MJ, Raizada MK. The future of hypertension therapy: sense, antisense, or nonsense? Hypertension 2001;37:357-64.
- Lin KF, Chao L, Chao J. Prolonged reduction of high blood pressure with human nitric oxide synthase gene delivery. Hypertension 1997;30:307-13.
- Kurogi Y. Mesangial cell proliferation inhibitors for the treatment of proliferative glomerular disease. Med Res Rev 2003;23:15-31.
- Parameswaran N, Nambi P, Brooks DP, Spielman WS. Regulation of glomerular mesangial cell proliferation in culture by adrenomedullin. Eur J Pharmacol 1999;372:85-95.
- Parameswaran N, Nowak W, Hall CS, Sparks HV, Spielman WS. Cellular and molecular actions of adrenomedullin in glomerular mesangial cells. Peptides 2001;22:1919-24.
- Chini EN, Choi E, Grande JP, Burnett JC, Dousa TP. Adrenomedullin suppresses mitogenesis in rat mesangial cells via cAMP pathway. Biochem Biophys Res Commun 1995;215:868-73.
- Sporn MB, Roberts AB. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol 1992;119:1017-21.
- Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996;45:522-30.
- Qi YF, Bu DF. Effects of different peptide fragments derived from proadreno-medullin on gene expression of adrenomedullin gene. Peptides 2002;23:1141-7.