Preoperative growth inhibition of human gastric adenocarcinoma treated with a combination of celecoxib and octreotide1
Introduction
Despite its decreasing trend over many decades, gastric cancer remains a major public health problem worldwide and accounts for 3%–10% of all cancer-related deaths[1]. Although adjuvant and neoadjuvant therapy may achieve moderate response rates, the regimens cannot produce complete responses in more than 10%–15% of patients, neither can they extend median survival beyond 1 year[2]. Therefore, it could be considered that most cytotoxic chemotherapies expose patients to the side-effects of a potentially ineffective treatment. When assessing the value of anticancer treatment, it is important to consider the impact on both survival and quality of life. This is particularly important for patients with advanced cancer whose life expectancy is short.
The current state of antigastric cancer requires further investigation of new therapeutic approaches, such as non-cytotoxic drugs for advanced gastric cancer. The inhibitive effect of aspirin or rofecoxib, a selective cyclooxygenase (COX)-2 inhibitor, on the proliferation of gastric cancer has been reported in our previous study[3]. These non-steroidal anti-inflammatory drugs (NSAIDs) exert dual function, the induction of NSAID-activated gene (NAG)-1 expression, and the inhibition of COX-2[4], which are widely overexpressed in gastric adenocarcinoma[5,6]. Various studies have demonstrated that the growth of normal cells and malignant tumors may be regulated by gut peptides[7,8]. Previous data have shown that the mean tumor volume in nude mice treated with octreotide, an analogue of somatostatin (SST), was significantly lower than that of the control group with an inhibitory rate of 60.6%[9,10]. The heterogeneity of SST receptors (SSTR) has been proven during the past decade by the cloning of different genes coding for SSTR subtype[11]. However, the positive rate of SSTR and the dominant SSTR subtype in gastric cancer remains unclear.
Some links between COX-2 and SST, such as extracellular signal-regulated protein kinase (ERK), a downstream molecule in the mitogen-activated protein kinase (MAPK) pathway induced by SSTR binding, have been indicated[12]. The hypothetically synergistic inhibition of the combination of 2 types of non-cytotoxic agents, rofecoxib and octreotide, on the growth of gastric cancer has also been confirmed in both in vitro and in vivo animal studies[13]. However, whether this regime could be beneficial for human gastric cancer treatment is still unknown. To address this issue, we investigated the effect of celecoxib alone or in combination with octreotide on the growth of gastric adenocarcinoma through a prospective, clinical randomized trial. The molecular targets for celecoxib and octreotide were also probed to understand the mechanism behind them.
Materials and methods
Patients This prospective study enrolled 75 consecutive patients (male: 46, female: 29; mean age: 55; range: 27–85) with histologically identified gastric adenocarcinoma who underwent surgery such as total gastrectomy, subtotal gastrectomy, or the extent of lymphadenectomy from March 2002 to December 2004 in West China Hospital, Sichuan University (Chengdu, China). The patients were randomized to receive different treatments before surgery: (i) celecoxib (Pfizer, New York, NY, USA) 0.2g/d,×7d, n=25; (ii) celecoxib 0.2 g/d, plus subcutaneous injection of octreotide (Novartis Pharma, Beijing, China) 100 µg/d, ×7 d, n=25; and (iii) preoperative treatment without any anticancer medicine as controls (n=25). No cytotoxic chemotherapy was applied. The data on gastric endoscopy, abdominal ultrasonography, magnetic resonance imaging (MRI), or computed tomography (CT) before the operation were matched as well as possible to get a parallel TNM stage (the Tumor, Lymph Node, Metastasis classification) among the three groups. This study was approved by the ethic committee of our hospital. All of the patients gave their informed consent for the preoperative treatments mentioned above.
Immediately after resection, the tissue samples of gastric adenocarcinoma were obtained from all of patients. One part of the tumor tissue was used for histological examination, while the other part was rapidly frozen at -80 °C.
Histopathological examination The tissue samples of gastric adenocarcinoma were fixed in 10% phosphate-buffered formalin, embedded in paraffin, and sectioned serially at a thickness of 4 µm. The tissue sections were deparaffinized and stained with hematoxylin-eosin (HE). The slides were examined by an assigned pathologist without knowledge of the group to which the specimen belonged. The degrees of tumor necrosis, infiltration of inflammatory cells, and fibrosis were semiquantitatively assessed in each tumor according to a 4-point arbitrary scale of + to ++++, that is, + is the inflammatory response less than 5% area; ++ is 5%–30% area; +++ is 30%–70% area; and ++++ is more than 70% area.
Apoptosis Terminal deoxynucleotide transferase-mediated dUTP nick end-labeling (TUNEL) assay[14] was performed using a commercial kit (Roche, Basel, Switzerland). The number of apoptotic cells positive for TUNEL staining was counted in the 10 arbitrary microscopic fields at a magnification of 400×per section. The apoptotic index was calculated as follows: apoptotic index (%)=(apoptotic cell number/total cell number)×100.
Immunohistochemistry for the detection of COX-2 and microvessel density The tissue sections were deparaffinized and rehydrated. The specimens were heated in an autoclave for 9 min at 90 °C for antigen retrieval, immersed in 0.3% hydrogen peroxide in methanol for 30 min, and then immersed in normal goat serum for 30 min to block endogenous peroxidase activity and the unspecific binding sites, respectively. Then, the tissue sections were incubated with a specific rabbit polyclonal antibody against human COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in a dilution of 1:100 overnight at 4 °C. The positive reaction was revealed by the streptavidin-biotin-peroxidase technique. The positive controls for the reaction were paraffin-embedded sections from colon carcinomas. Non-immune rabbit serum was used as a negative control.
COX-2-positive cells were counted from 500 cells on 5 fields at a magnification of 400×, with a 40×objective lens and a 10×ocular lens of 0.22 mm2 per field. The intensity of COX-2 staining was graded on a scale of 0–2 (0, negative; 1, moderate; and 2, strong intensity of staining). The percentage of stained cells among the total tumor cells were assessed as 4 grades (0, ≤5%; 1, 6%–25%; 2, 26%–50%; and 3, ≥51%). Furthermore, the tumors defined as COX-2 positive were semiquantitatively scored as (–) 0–1, (+) 2–3, (++) 4–6, and (+++) >6 on the basis of the final product of intensity score time percentage score.
The immunohistochemical visualization of microvessel density (MVD) was carried out as mentioned above. The specific antibody against human factor VIII related antigen was purchased from Zymed (San Francisco, CA, USA). The microvessels were defined as any factor VIII related antigen positive endothelial cell or endothelial cell cluster with or without a visible lumen. The locations where we measured the MVD were determined according to previous studies[15,16]. The marginal areas of the largest transect of tumor where the progressive malignant tissues are found are usually companied with flourishing angiogenesis. The microvasculature developed in tumors as an intense network and each distinct branch was interpreted as a single vessel. Large sinusoidal vessels were also counted as a single vessel. Positive controls for the reaction were paraffin-embedded sections from vessel tissues. Non-immune rabbit serum was used as a negative control.
Individual microvessels were counted on 5 fields at a magnification of 400× in a highly vascular tumor area (hot spot). The microvessel count was performed in the tumor, excluding areas with prominent hyalinization and necrosis. The mean of 5 counts was used as the MVD in each case. Data were expressed as the number of vessels per mm2.
NAG-1 gene detection by RT-PCR Total RNA was extracted from tumor tissues using the Tripure RNA isolation kit (Roche, Basel, Switzerland). The primers (Shanghai Sangon, Shanghai, China) designed for PCR were according to the sequences on GeneBank. The sequences of primers were as follows: NAG-1, sense: 5'-TTGCGGAAACGCTACGAG-3', antisense: 5'-AACAGAGCCCGGTGAAGG-3'; and GAPDH, sense: 5'-ACCACAGTCCATGCCATCAC-3', antisense: 5'-TCCACCACCCTGTTGCTGTA-3'.
Using the TaKaRa one-step method (TaKaRa, Dalian, China) for RT-PCR, the reaction system (12.5 µL for the biopsy, 25 µL for the surgery samples) was as follows: 3.75 µL RNase-free dH2O, 1.25 µL 10×one-step RNA PCR buffer, 1.25 µL (10 mmol/L) dNTP mixture, 2.5 µL (25 mmol/L) MgCl2, 0.25 µL (40 U/µL) RNase inhibitor, 0.25 µL (5 U/µL) AMV RTase XL, 0.25 µL (5 U/µL) AMV-optimized Taq, 0.5 µL (20 µmol/L) sense primer, 0.5 µL (20 µmol/L) antisense primer, and 2 µL total RNA. The reaction conditions were: 55 °C for 30 min; 94 °C for 2 min; 94 °C for 10 s, 57 °C for 10 s, 72 °C for 10 s, 30 cycles; and 72 °C for 3 min. The amplification products of NAG-1 and GAPDH were 209 and 452 bp, respectively. The gray scale integral ratio of NAG-1/GAPDH was regard as the relative transcription level of NAG-1, and the quantitative analysis of amplification products was performed by using the gelatin imaging system (Kodak, Rochester, NY, USA).
Transcription of SSTR-1, SSTR-2, and SSTR-3 in gastric cancer with real-time fluorescence-quantitative RT-PCR Total RNA was extracted from tumor tissues using the Tripure RNA isolation kit (Roche, USA). First-strand cDNA was produced by Moloney murine leukemia virus reverse transcriptase (MBI, USA) with 1 µg of total RNA and 0.5 g of oligo (dT) 18. The GAPDH gene was used as an internal control. TaqMan (Shanghai Sangon, Shanghai, China) probe-based real-time fluorescence-quantitative RT-PCR was used for quantification. PCR was performed in a reaction system as follows: 1×PCR buffer, 2.5 mmol/L MgCl2, 0.3 mmol/L dNTP, 2.5 µmol/L each of the upstream and downstream primers for SSTR-1/GAPDH, SSTR-2/GAPDH, and SSTR-3/GAPDH, 2.5 µmol/L TaqMan probe, 1.5 U heat-resistant Taq DNA polymerase, 14.8 µL ddH2O, and 2.5 µL cDNA. After denaturation for 2 min at 94 °C, PCR was started. One cycle consisted of a denaturation step for 20 s at 94 °C, an annealing step for 30 s at 53 °C (gathering fluorescence), and an extension step for 60 s at 60 °C. After 45 cycles, the values of cycle threshold for SSTR-1, SSTR-2, SSTR-3, and GAPDH of all the samples were measured. Using the comparative threshold method, the relative quantity was calculated, equal to the Ct of SSTR-1, SSTR-2, or SSTR-3 divided to the Ct of GAPDH. The sequences of the primers used were as follows: SSTR-1, sense: 5'-CCACGGTGAGTCAGCTGT-3', antisense: 5'-GAAAGAGCGCTTGAAGTTGTCT-3'; SSTR-2, sense: 5'-CCAGCCCTTAAAGGCATGTT-3', antisense: 5'-CTTGA-CCAAGCAGAGGACA-3'; SSTR-3, sense: 5'-TGCCTTC-TTTGGGCTCTACT-3', antisense: 5'-CTGCTTGAAGCGG-TAGGAGA-3'; GAPDH, sense: 5'-GGGTGTGAACCATGA-GAAGT-3', antisense: 5'-CCAAAGTTGTCATGGATGACCT-3'.
Detection of SSTR-2 in tissue sample with biomolecular interaction analysis system[17] The tissue samples of gastric cancer or no-cancer gastric mucosa were broken to pieces with scissors on ice before the addition of special buffer (4 µL/mg tissue) supplied by BIAcore AB (Amersham Biosciences, Uppsala, Sweden) for the extraction of proteins. Then the samples were homogenized in ice-cold phosphate buffered solution and centrifuged to pellets. The protein concentration of the supernatants was measured with Pierce BCA (bicinchoninic acid) protein assays (Pierce Biotechno-logy, Rockford, IL, USA), and the supernatants were stored at -70 oC.
The SSTR-2 antibody was immobilized on BIAcore CM5 sensor chip surface using an amine coupling kit (BIAcore AB). A biomolecular interaction analysis system (BIAcore X, Amersham Biosciences, Sweden) was used to measure the binding capacity between SSTR-2 and its antibody. The 60 µL samples were then injected onto the chip immobilized with the SSTR-2 antibody at a flow rate of 30 µL/min; the buffer for the extraction of proteins was used for blank subtraction. The detection of SSTR-2 was performed in a BIA (biomolecular interaction analysis) system through binding–binding/dissociation–washing–regenerations. The raw data from individual binding experiments were determined using BIA evaluation software (Amersham Biosciences, Sweden). The technological fluctuations of the baseline were ±5 resonance units (RU). The data were normalized to RU/mg protein. Specific binding=total binding-non-specific binding.
Statistical analysis All the results were expressed as the mean ± SD and were analyzed by SPSS 11.0 for Windows software (SPSS, Chicago, IL, USA). Statistical significance was calculated with one-way ANOVA and χ2-test. Differences were considered statistically significant at P<0.05.
The sample size was evaluated with MVD data according to the following formula, where α=0.05 and β=0.10:
The calculation indicated that the proper sample size should be at least 23 patients per group and 69 patients in the 3 groups. The present study enrolled 25 patients into each group with a strong power of test (1-β=0.90).
Results
TNM staging and histopathological examination of gastric adenocarcinomas The percentage of TNM stage II, III, and IV in 75 cases was 13.3%, 45.3%, and 41.3%, respectively. According to World Health Organization histological grouping[18], the tumors were divided into the well-differentiation group (13.3%), moderately-differentiation group (40.0%), and poor differentiation group (46.7%). The histological types of gastric adenocarcinomas in this study included papillary, tubular, mucinous adenocarcinoma, and signet-ring cell carcinoma.
The scores of tumor necrosis and fibrosis in 75 cases are listed in Table 1. Compared with the control group, tumor necrosis in the celecoxib group had no marked change, but it was significantly increased in the combination group (Table 1, P<0.05). Furthermore, the semiquantitative analysis also showed an upregulated fibrosis level in the combination group (Figure 1, P<0.05). The infiltration of inflammatory cells in each group did not show significant differences. The tumor differentiation type in the control group did not bear an obvious relationship to the extent of necrosis or fibrosis (r=0, P>0.05).
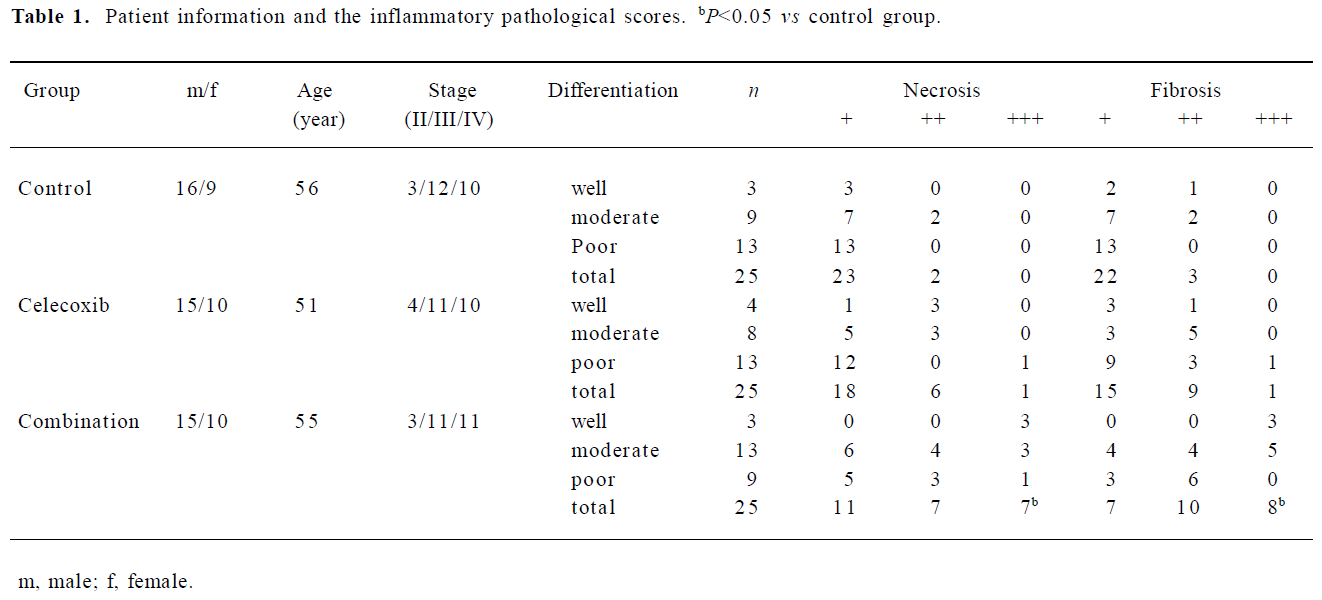
Full table

Apoptosis A significantly increased apoptotic rate was observed in the combination group (P<0.05), but not in the celecoxib group (Figure 2; Table 2).


Full table
MVD FVIII-R Ag-positive endothelial cells or endothelial cell clusters in the combination group were less than those in celecoxib or control groups (Figure 3). The data from semiquantification of MVD per square in the tissue sections of gastric cancer also showed a significant decrease in the combination group (P<0.05), while no marked change was observed in the celecoxib group (Table 2).

COX-2 expression Although the COX-2-positive staining in the combination or celecoxib groups seemed lighter than that in the control group (Figure 4), semi-quantification did not show a significant difference between them (Table 2; P>0.05).

NAG-1 expression The transcription of NAG-1 was detected in the gastric carcinomas of each group and were upregulated in the celecoxib and combination groups (P<0.05). Surgery itself did not affect the transcription of NAG-1 in the tissues of gastric carcinoma.
Transcription of SSTR-1, SSTR-2, and SSTR-3 in gastric cancer determined by real-time fluorescence-quantitative RT-PCR The relative transcription levels of SSTR-1, SSTR-2, and SSTR-3 in gastric adenocarcinomas were 14.86±9.71, 39.08±29.65, and 19.74±16.93, respectively. The expression of SSTR-2 in gastric adenocarcinoma was highest among the SSTR subtypes.
Detection of SSTR-2 in gastric tissue The biomolecular interaction analysis system revealed that SSTR-2 proteins in gastric cancer before surgery were greatly higher than that either in gastric mucosa or in surgical specimens of gastric cancer (P<0.01). However, after surgery, the expression of SSTR-2 in celecoxib group was significantly higher than that of control group (P<0.05). The addition of octreotide before surgery did not seem to enhance SSTR-2 expression comparing with celecoxib group (Table 3, P>0.05). No relationship between the expression of SSTR-2 and the differentiation of gastric carcinomas was observed. However, more necrosis and fibrosis were found in the SSTR-2-positive tumors than in the SSTR-2-negative ones treated with celecoxib and octreotide (Table 4).

Full table

Full table
Adverse events of treatment During the study, no severe adverse events, such as gastrointestinal bleeding, perforation, diarrhea, and cardiovascular events, were recorded in the treatment groups. There were also no notable increases of alanine aminotransferase or aspartate aminotransferase (approximately 3 or more times the upper limit of normal) reported. The preoperative administration of celecoxib and octreotide did not result in increased bleeding in gastrectomy and retard of wound healing.
Although more necrosis lesions were found in the combination group, the patients did not present fever, abdominal pain, or massive upper gastrointestinal bleeding.
Discussion
The inhibitive effects of the combination of non-cytotoxic drugs (COX-2 inhibitor and SST analogue) on gastric cancer in vitro or in vivo have been reported in animal experiments[9,13]. In the present study, more necrosis was detected in gastric adenocarcinomas of patients treated with celecoxib and octreotide for 1 week. Well-matched tumor stage among the three groups suggested that these histopathological changes might not be due to the unbalance of tumor size among the groups. Usually the poorly differentiated gastric adenocarcinomas may present more necrosis because of unparallel angiogenesis to flourishing tumor cells. However, the higher score of necrosis was presented in the combination group with well or moderate differentiated gastric adenocarcinomas. It indicated an inhibitive effect of the combination of two non-cytotoxic drugs on the tumors. The proliferation and infiltration of tumor cells rely on the development of blood vessels of tumors[19]. Celecoxib combined with octreotide significantly decrease the MVD of the tumors.It might be the cause of necrosis lesions described above.
In addition, the fibrosis of tumors was scant in either the control or celecoxib groups, but was extensively increased in the combination group. This significant difference between the groups might suggest the effect of treatment with celecoxib and octreotide. It is unclear whether or not it could be regarded as a consequence of massive necrosis or a direct reaction to the combination treatment. Moreover, it has been observed that the desmoplastic reaction in gastric adenocarcinomas may vary with the tumors from scant to abundant but with unclear mechanisms[20]. It was impossible to enroll patients according to the extension of stroma before the surgery due to limited biopsies. Therefore, more fibrosis in the combination group still could not exclude the result of unparallel grouping on the extension of stroma before the operation.
The growth characteristics of tumor cells consist of unlimited proliferation, imbalance between generation and apoptosis of cells, and the ability of invasion[21].The apoptotic rate of gastric adenocarcinoma cells was significantly increased in the combination group by which the growth of gastric cancer would be arrested efficiently in a peaceful way.
The effects of celecoxib on human gastric adenocarcinomas tentatively suggested that COX-2 might be the target of celecoxib. However, COX-2 expression in tumors in the combination or celecoxib groups did not decrease when compared with the control group. NAG-1, a non-COX-2 pathway, is considered a downstream target protein of early growth response-1 gene with an inhibitive ability against cancer[22]. It was obviously upregulated in the human gastric adenocarcinomas treated with celecoxib in this study. Therefore, the inhibitive effects of celecoxib on the growth of gastric cancer were mediated through increasing NAG-1.
It has been reported that the expression of SSTR-3 was significantly lower in gastric cancer when compared with normal mucosa[23]. In the present study, although mRNA for SSTR-1, SSTR-2, and SSTR-3 were detected in the tissues of gastric adenocarcinomas, the quantity of SSTR-2 mRNA was the highest. Based on this result, the SSTR-2 protein measured by the biomolecular interaction analysis system was compared among the 3 groups. Both the positive rate and quantity of SSTR-2 in the biopsy specimens of gastric cancer were significantly higher than those in the biopsy specimens of gastric mucosa with superficial gastritis. Theoretically, all of gastric mucosa should be SSTR-2 positive. The low positive rate and quantity of SSTR-2 in the biopsy specimens of gastric mucosa of the present study may be related to superficial biopsy without including enough gastric crypts where SSTR-2 is located intensively. Therefore, it is still difficult to decide whether the SSTR-2 protein was really increased in the malignant tissues. Even though, this result indicated that only about one-third of patients with gastric cancer have molecule targets for the direct effect of octreotide.
The surgical removal of gastric cancer was very crucial to the treatment. However, the SSTR-2 protein in the surgical specimens of gastric cancer was greatly lower than that in the pre-operation specimens of gastric cancer. It meant that the operation itself might mask the expression of the SSTR-2 protein which might be a negative control of cancer cell growth, bringing easy proliferation and metastasis of tumors. Interestingly, celecoxib presented the ability to induce the expression of the SSTR-2 protein in gastric cancer in this study. It was helpful for octreotide to target the cancer cells. The pre-operation treatment with celecoxib plus octreotide for the patients with gastric cancer may be a valuable recommendation.
In this study, we also found that octreotide could partly inhibit the proliferation of SSTR-negative neoplasms. These results suggest that octreotide may indirectly affect tumor growth through the inhibition of release of some peptides or growth factors such as insulin, epidermal growth factor, and insulin-like growth factor-1, which promote tumor proliferation[24]. Beside the synergic antitumor mechanisms discussed above, both the COX-2 inhibitor and octreotide also could inhibit the intracellular mitogen-activated protein kinase signaling pathways, and therefore, their combination in a regime may greatly suppress the synthesis of DNA in tumor cells[25,26].
COX catalyzes the initial step of arachidonic acid metabolism and prostaglandin production. COX activity has been found to be associated with 2 distinct isoenzymes: COX-1 and COX-2. COX-1 was hypothesized to be involved in the protection of gastric mucosa and the maintenance of hemostasis, whereas COX-2 was thought to be involved in pathophysiological processes, such as inflammation and the proliferation of cells[27]. Whole-blood assays for celecoxib have shown COX-2/COX-1 selectivity ratios as 7.6[28]. The possible injury of mucosal defense caused by celecoxib was expected to be diminished by octreotide with which the output of gastric acid is able to be inhibited through the mediation of SSTR in parietal cells of gastric mucosa. Interestingly, the present regime displayed a substantial anti-proliferation of gastric cancer without severe adverse events owing to the synergic actions on both the curative and side-effects.
Several studies have demonstrated that patients with gastric adenocarcinomas receiving preoperative chemotherapy had a downstaging of tumor size and an increase in rates of curative resection. Nevertheless, the overall survival has not been prolonged. In addition, the implementation of that treatment has posed several problems with respect to the toxicity of the treatment, depression of anti-tumor immune, and a high rate of locoregional recurrence[29,30]. This prospective study suggested that the combination of celecoxib and octreotide resulted in the up-regulation of anticancer approaches, including NAG-1 and SSTR-2 and a substantial pathological response in patients with gastric cancer. As the non-cytotoxic agents, celecoxib combined with octreotide was well-tolerated and safe when followed by gastrectomy. This strategy is worthy of further clinical trials to evaluate its benefit on the rates of curative resection and relapse on overall survival.
References
- Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005;241:27-39.
- Wohrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol 2004;15:1585-95.
- Liu CL, Tang CW, Wang CH, Zhou XC. The effects of selective cyclooxygenase-2 inhibitors on the growth of gastric adeno-carcinoma. J West China Univ Med Sci 2003;3:372-5. Chinese..
- Baek SJ, Wilson LC, Lee CH, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclo-oxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther 2002;301:1126-31.
- Uefuji K, Ichikura T, Mochizuki H. Expression of cyclo-oxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol 2001;76:26-30.
- Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, et al. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer 2001;91:1876-81.
- Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer 1999;80:448-54.
- Pollak MN, Schally AV. Mechanisms of antineoplastic action of somatostatin analogs. Proc Soc Exp Biol Med 1998;217:143-52.
- Tang C, Wang C, Tang L. Effects of combined octreotide and aspirin on the growth of gastric cancer. Chin Med J (Engl) 2003;116:373-7.
- Pinski J, Halmos G, Yano T, Szepeshazi K, Qin Y, Ertl T, et al. Inhibition of growth of MKN45 human gastric-carcinoma xenografts in nude mice by treatment with bombesin/gastrin-releasing-peptide antagonist (RC-3095) and somatostatin analogue RC-160. Int J Cancer 1994;57:574-80.
- Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev 1995;16:427-42.
- Kwon KS, Chae HJ. Sodium salicylate inhibits expression of COX-2 through suppression of ERK and subsequent NF-kappaB activation in rat ventricular cardiomyocytes. Arch Pharm Res 2003;26:545-53.
- Tang CW, Liu CL, Zhou XC, Wang CH. Enhanced inhibitive effects of combination of rofecoxib and octreotide on the growth of human gastric cancer. Int J Cancer 2004;112:470-4.
- Gavrieli Y, Sherman Y, Beb-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493-501.
- Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer 2002;86:1566-77.
- Dhar DK, Wang TC, Tabara H, Tonomoto Y, Maruyama R, Tachibana M, et al. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res 2005;11:6472-8.
- Casper D, Bukhtiyarova M, Springman EB. A Biacore biosensor method for detailed kinetic binding analysis of small molecule inhibitors of p38alpha mitogen-activated protein kinase. Anal Biochem 2004;325:126-36.
- Sarbia M, Becker KF, Hofler H. Pathology of upper gastrointestinal malignancies. Semin Oncol 2004;31:465-75.
- Jazowiecka-Rakus J, Jarosz M, Kozlowska D, Sochanik A, Szala S. Combination of vasostatin and cyclophosphamide in the therapy of murine melanoma tumors. Acta Biochim Pol 2007;54:125-33.
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma –– 2nd English Edition. Gastric Cancer 1998;1:10-24.
- Diasio RB, Fourie J. Targeting the epidermal growth factor receptor in the treatment of colorectal cancer: state of the art. Drugs 2006;66:1441-63.
- Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol 2005;67:356-64.
- Hu C, Yi C, Hao Z, Cao S, Li H, Shao X, et al. The effect of somatostatin and SSTR3 on proliferation and apoptosis of gastric cancer cells. Cancer Biol Ther 2004;3:726-30.
- Kouroumalis EA. Octreotide for cancer of the liver and biliary tree. Chemotherapy 2001;47 Suppl 2:150-61.
- Wang CH, Tang CW, Liu CL, Tang LP. Inhibitory effect of octreotide on gastric cancer growth via MAPK pathway. World J Gastroenterol 2003;9:1904-8.
- Liu C, Tang C, Zhou X, Wang C. Enhanced inhibitive effects of combination of rofecoxib and octreotide on the growth of human gastric cancer. Basic Med Sci Clin 2004;24:277-81. Chinese..
- Gajraj NM. Cyclooxygenase-2 inhibitors. Anesth Analg 2003;96:1720-38.
- Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther 2001;296:558-66.
- Macdonald JS. Clinical overview: adjuvant therapy of gastrointestinal cancer. Cancer Chemother Pharmacol 2004;54 Suppl 1:S4-11.
- Lindsey H. Preoperative chemoradiotherapy shows promise in gastric cancer. Lancet Oncol 2004;5:519.
