Inhibitory effect of dendroaspis natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscles of guinea pigs1
Introduction
Natriuretic peptides (NP) are a polypeptide family with many bioactivities and physiologically function as hormones or neurotransmitters[1]. Up to now, NP include the atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP) and dendroaspis natriuretic peptide (DNP), Micrurus natriuretic peptide, and ventricular natriuretic peptide. They are distributed all over the body and they have natriuretic-diuretic[2] and vasorelaxation[3] functions designed to lower blood pressure[4] and control electrolyte homeostasis[5]. Since immunoactivity of NP was found in the stomach and small intestines of rats, the investigation of the relationship between NP and the gastrointestinal function was preluded[6]. The studies about the physiological functions of NP have mainly focused on gastric absorption[7], secretion[8], and intestinal motility[9], but there are few of NP studies about the regulation of gastric smooth muscle motility.
Our previous study[10] indicated that DNP inhibited gastric motility in the gastric antrum of guinea pigs. DNP, a 38 amino acid peptide, was isolated from the venom of the green mamba. It has structural and functional similarities to other members of the NP family. Studies about the physiological function of DNP have mainly focused on the cardiovascular system[11], urinary system[12], and genitals[13]. Study about the relationship between DNP and gastrointestinal functions are few. Kim et al[14] demonstrated that the DNP system presents in the rat colon and regulates colonic motility as a local regulator. It is the first report to investigate the relationship between DNP and gastrointestinal functions.
Up to now, there is no report of the relationship between DNP and gastric motility. The aim of present study was to determine whether the NP receptor (NPR) is present in the stomach of guinea pigs and to investigate the effect of DNP on the gastric motility of guinea pigs and its mechanism.
Materials and methods
Strip preparation EWG/B guinea pigs of either sex (weighing 300±50 g) and bred by the Experimental Animal Center of Dalian Medical University(Dalian, China) were euthanized by a lethal intravenous dose of pentobarbital sodium (50 mg/kg).The abdomen of each rat was opened along the midline, and the stomach was removed and placed in pre-oxygenated Tyrode’s solution at room temperature. The mucous layer was removed and strips (approximately 2.0 mm×15.0 mm) of gastric antral circular muscles were prepared. The longer axis of the stomach was cut parallel to the circular muscle fibers. The muscle strips were placed in a chamber. One end of the strip was fixed on the lid of the chamber through a glass claw; the other end was attached to an isometric force transducer (TD-112S, Nihon Kohden-Kogyo Japan) to record contractions. The chamber (2 mL volume) was constantly perfused with pre-oxygenated Tyrode’s solution at 1 mL/min. The temperature was maintained at 37.0±0.5 °C by a water bath thermostat (WC/09-05, Chongqing, China). The muscle strips were allowed to incubate for at least 40 min before the experiments were started.
Autoradiograph of the NPR method[15] The iodinated DNP was prepared by the modified chloramine-T method, divided, and kept at -70 °C until use. The iodinated DNP was repurified by HPLC, and the specific activity was approximately 2000 Ci/mmol. The anti-DNP antibody was purchased from Peninsula Laboratories (San Carlos, Californai, USA). The lyophilized samples were reconstituted with phosphate buffer (pH 7.4) containing 50 mmol/L NaCl, 0.1% bovine serum albumin (BSA), 0.1% Triton X-100, and 0.01% sodium azide. Synthetic DNP was used as a standard. After incubation with the anti-DNP antibody for 24 h at 4 °C, approximately 15 000 cpm of 125I-DNP was added, and the samples were incubated again for an additional 24 h at 4 °C. When 125I-DNP bonded with its receptor thoroughly, the tissue was covered by photographic emulsion. At the site of the radioactive material, the radioactive emission acts on the silver halide in the emulsion. Subsequent development and fixation turned the radiated silver halide into black grains.
Cell preparation and electrophysiological recording Guinea pigs of either sex weighing 250–350 g were euthanized by a lethal intravenous injection of pentobarbital sodium (50 mg/kg). The antral part of the stomach was rapidly cut, then the mucosal layer was separated from the muscle layers. The longitudinal layer of muscle was then dissected from the other muscle layers using fine scissors and then cut into small segments (1 mm×4 mm). These segments were kept in modified Kraft–Bruhe (K–B) medium at 4 °C for 15 min. Then they were incubated at 36 °C in 4 mL of digestion medium (Ca-free physiologic salt solution [Ca-free PSS]) containing 0.1% collagenase II, 0.1% dithioerythritol, 0.15% trypsin inhibitor, and 0.2% BSA for 25–35 min. The softened muscle segments were transferred into the modified K–B medium, and the single cells were dispersed by gentle trituration with a wide-bore, fire-polished glass pipette. The isolated gastric myocytes were kept in modified K–B medium at 4 °C until they were ready for use.
Isolated cells were transferred to a 0.1 mL chamber on the stage of an inverted microscope (IX-70 Olympus, Tokyo, Japan) and allowed to settle for 10–15 min. The cells were continuously perfused with a isosmotic PSS at a rate of 0.9–1.0 mL/min. An 8-channel perfusion system (L/M-sps-8, List Electronics, Berlin, Germany) was used to exchange the solution. The calcium-activated potassium currents (IK[ca]) was recorded using the conventional whole cell patch-clamp technique[16,17]. Patch-clamp pipettes were manufactured from borosilicate glass capillaries (GC 150T-7.5, Clark Electro-medical Instruments, London, UK) using a 2-stage puller (PP-83, Narishige, Tokyo, Japan). The resistance of the patch pipette was 3–5 MΩ¸ when filled with pipette solution. Liquid junction potentials were canceled prior to the seal forma-tion. Whole-cell currents were recorded with an Axopatch 1-D patch-clamp amplifier (Axon Instruments, Foster City California, USA), and data were filtered at 1 KHz. Command pulses, data acquisition, and storage were applied by using the IBM-compatible, 486-grade computer and pCLAMP 6.02 software (Axon Foster City, California, USA). Spontaneous transient outward currents were recorded simultaneously by an EPC-10-HEAKA amplifier (HEAKA Instruments, Berlin, Germany). All experiments were performed at room temperature (20–25 °C).
Drugs and solutions Tyrode’s solution containing (in mmol/L) NaCl 147, KCl 4, MgCl2·6H2O 1.05, CaCl2 ·2H2O 0.42, Na2PO4·2H2O 1.81, and 5.5 mmol/L glucose was used. Ca2+-free PSS containing (in mmol/L) NaCl 134.8, KCl 4.5, glucose 5, and N-(2-hydroxyethyl) piperazine-N-(2-ethanesulphonic acid) (HEPES) 10 was adjusted to pH 7.4 with Tris (hydroxy-methyl) aminomethane. Modified K–B solution containing (in mmol/L) L-glutamate 50, KCl 50, taurine 20, KH2PO4 20, MgCl2·6H2O 3, glucose 10, HEPES 10, and egtazic acid 0.5 was adjusted to pH 7.40 with KOH. PSS containing (in mmol/L) NaCl 134.8, KCl 4.5, MgCl2·6H2O 1, CaCl2 ·2H2O 2, glucose 5, HEPES 10, and sucrose 110 was adjusted to pH 7.4 with Tris. In order to abolish delayed rectifier potassium currents (IK[V]), external solution contained 4-aminopyridine 10 mmol/L, a selective inhibiter of IK(V). The pipette solution recording IK(ca) contained (in mmol/L) potassium-aspartic acid 110, Mg-ATP 5, HEPES 5, MgCl2·6H2O 1.0, KCl 20, egtazic acid 0.1, di-tris-creatine phosphate 2.5, and disodium-creatine phosphate 2.5; its pH was adjusted to 7.3 with KOH. Tetraethylammonium (TEA), DNP, LY83583, and zaprinast were made up as stock solutions. All chemicals in this experiment were purchased from Sigma (St Louis, MO, USA).
Data analysis All data was expressed as mean±SD. Statistical significance was evaluated by a t-test. Differences were considered to be significant when a P-value was less than 0.05.
Results
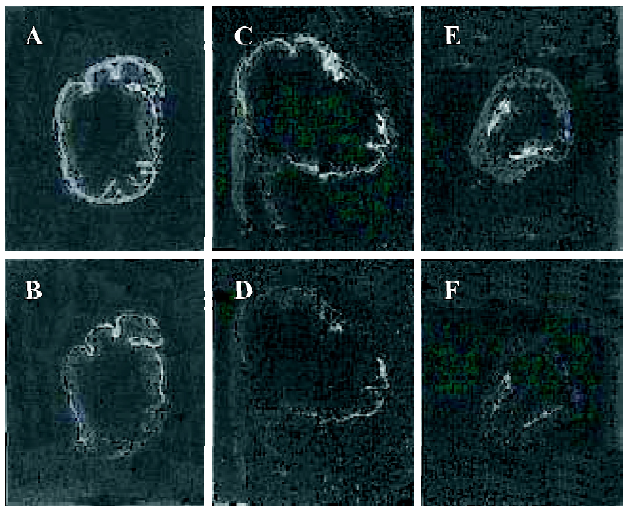
Distribution of the NPR in the stomach wall of guinea pigs Using the radioautograph technique, the distribution of the NPR in the different regions of the stomach of guinea pigs was detected. The NPR existed both in the mucosal layer and muscle layer, and the distribution order of the NPR in density was: antrum>body>fundus in the muscle layer (n=6, Figure 1)
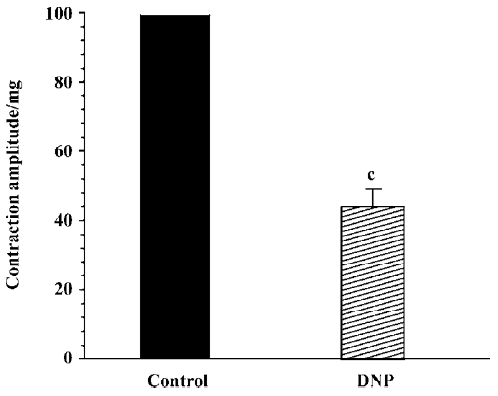
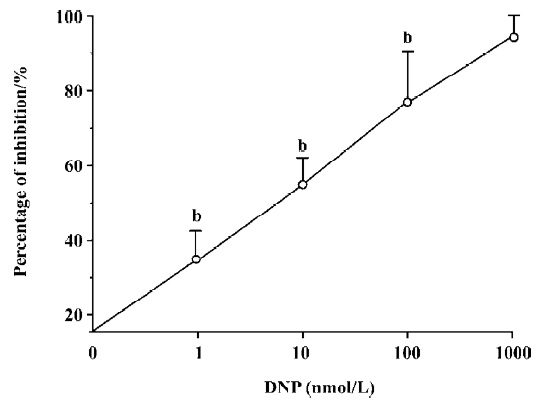
Effect of DNP on spontaneous contraction in antral circular smooth muscles of guinea pigs The spontaneous contraction usually appeared approximately 40 min after incubating the muscle strips in Tyrode’s solution. The effects of 10 nmol/L DNP on spontaneous contraction in gastric antral circular smooth muscles of guinea pigs were observed. After administering DNP, the spontaneous contraction was significantly inhibited. (n=6, Figure 2). Different concentrations of DNP obviously inhibited spontaneous contraction in a dose-dependent manner, and the inhibition percentages was 35%±7%, 54%±8%, 78%±13%, and 94%±6% at 1, 10, 100, and 1000 nmol/L respectively (n=6, Figure 3). High concentration (1000 nmol/L) of DNP completely inhibited spontaneous contraction and shifted down the base line.
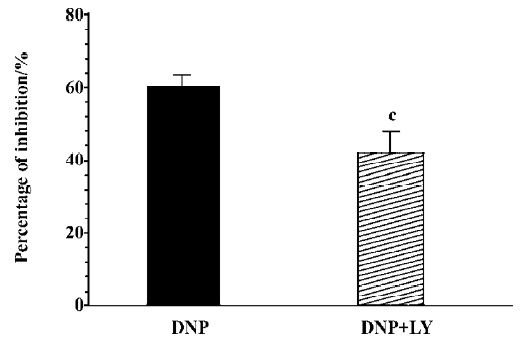
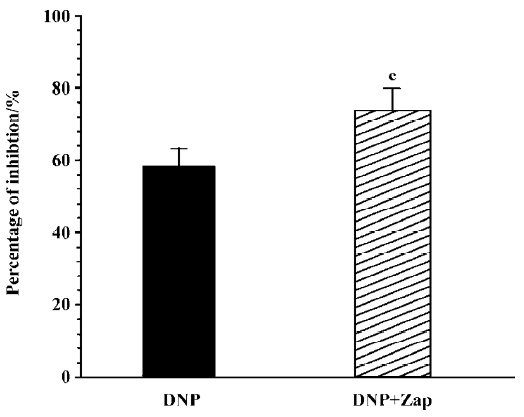
Effect of LY83583 and zaprinast on DNP-induced inhibition To further investigate the mechanism by which DNP inhibited gastric motility in guinea pigs, the effect of DNP on gastric motility was observed in the condition of administering LY83583, an inhibitor of guanylate cyclase, and zaprinast as a phosphoesterase inhibitor to change the production of cGMP. LY83583 (10 nmol/L) markedly diminished the inhibitory effect of DNP on spontaneous contraction, but could not completely abolish the inhibitory effect (n=8, Figure 4). Zaprinast (100 nmol/L) potentiated the inhibitory effect of DNP on spontaneous contraction (n=8, Figure 5).
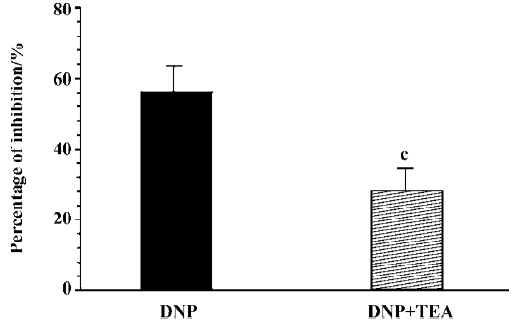
Effect of DNP on spontaneous contraction in the presence of TEA It is well known that nitric oxide (NO) and NP are cGMP generation systems in the body. Our previous studies demonstrated[16,17] that sodium nitroprusside, a NO donor, inhibited the spontaneous contraction of gastric smooth muscles via increasing IK(ca). So in the present study, the effect of TEA, a non-selective potassium channel blocker, on the DNP-induced inhibitory effect on gastric motility was observed. After the muscle strips were pretreated with TEA (10 mmol/L), the inhibitory effect of DNP (10 nmol/L) on spontaneous contraction was significantly diminished (n=6, Figure 6).
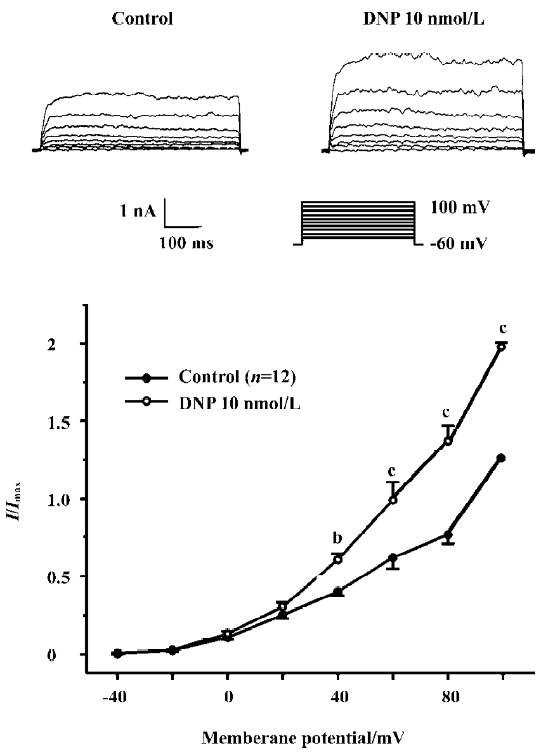
Effect of DNP on IK(ca) Under the conventional whole cell patch-clamp mode, the membrane potential was clamped at -60 mV, and the IK(ca) was elicited by step voltage command pulse from -40 mV to 100 mV for 400 ms with a 20 mV increment at 10 s intervals. 10 nmol/L DNP (n=6, Figure 7) markedly increased the IK(ca), and the increasing amplitude was 62.31%±3.22% at 60 mV.
Discussion
Our present study clearly shows that the NPR existed in the stomach of guinea pigs and its density was largest in the antrum. DNP, a new member of the NP family, inhibited the motility of gastric antral smooth muscles in a dose-dependent manner. The DNP-induced inhibitory effect was diminished by LY83583, an inhibitor of guanylate cyclase, and was potentiated by zaprinast, a phosphoesterase inhibitor. TEA, a non-selective potassium channel blocker, also suppressed the DNP-induced inhibitory effect. DNP increased IK(ca) in a dose-dependent manner in the gastric circular myocytes of guinea pigs.
The NPR was extensively distributed in many tissues and systems; for example, in the rat brain[18], in the kidney of the Japanese eel (Anguilla japonica)[19], in the guinea pig cecum[20], and in the rat heart [21]. In the present study, we found that the NPR also exists in the stomach of guinea pigs and the density is largest in the gastric antrum. There are many reports about ANP, BNP, and CNP, however, studies about DNP are few. The studies about DNP have focused on the cardiovascular system. Ha et al[22]suggested that DNP protects postischemic myocardial injury by an increased ratio of Bcl-2 to the Bax protein after ischemia-reperfusion. Ha et al[23] demonstrated that DNP induces the apoptosis of cardiac muscle cells. Kim et al[14] demonstrated that the DNP system presents in the rat colon and regulates colonic motility as a local regulator. Kim et al’s report was the first report about the relationship between DNP and gastrointestinal functions. In present study, DNP significantly inhibited the spontaneous contraction of gastric antrum in guinea pigs.
NP are similar to NO, which is a cGMP generation system in the living body. Their physiological functions are very important in life science. Ha et al[23] demonstrated that DNP may induce the apoptosis of muscle cells by the cGMP pathway. However, Singh et al[11] reported that vasodilatation in human mammary glands was predominantly mediated via the direct activation of smooth muscle NPR-A. It is probable that there is a direct and indirect activation of NPR to exert the physiological function of DNP. Up to now, there are only a few studies investigating the mechanism of DNP action, so the key point of this study was to determine the relationship between DNP-induced inhibition and cGMP in gastric antral circular smooth muscles. In present study, the DNP-induced inhibition was diminished by LY83583, an inhibitor of guanylate cyclase, and was potentiated by zaprinast, a phosphoesterase inhibitor. Our study indicated that the DNP-induced inhibitory effect on the spontaneous contraction of gastric antral smooth muscles was mediated by cGMP in guinea pigs.
In our present study, TEA weakened the effect of the DNP-induced inhibition on spontaneous contraction. It is well known that the potassium channel has a direct relationship to the relaxation of smooth muscles. There are 2 kinds of potassium currents, IK(ca) and delayed rectified potassium currents in the gastric antral smooth muscle cells of guinea pigs[24,25]. The present study demonstrated that DNP increased IK(ca) in a dose-dependent manner. It could be concluded that the potassium channel participated in the DNP-induced relaxation. Many previous studies also reported that the potassium channel is involved in the NP-induced regulation of many physiological functions. Van der Zander et al[25] demonstrated that NO and IK(ca) regulated the effect of the BNP-induced relaxation on vascular smooth muscles. Up to now, there is no report about the relationship between DNP and ion channels. Our present study indicates that DNP inhibits gastric motility by activating the IK(ca).
In summary, our study confirms that the NPR system exists in the stomach of guinea pigs, and the density of the NPR is hightest in the antrum. DNP inhibits gastric motility of circular smooth muscles. The DNP-induced inhibition enhances cGMP production, and IK(ca) may be involved in this process.
References
- Tingberg E, Roijer A, Thilen U, Ohlin H. Neurohumoral changes in patients with left ventricular dysfunction following acute myocardial infarction and the effect of nitrate therapy: a randomized, double-blind, placebo-controlled long-term study. J Cardiovasc Pharmacol 2006;48:166-72.
- Alvelos M, Ferreira A, Bettencourt P, Pimenta J, Azevedo A, Serrao P, et al. Effect of saline load and metoclopramide on the renal dopaminergic system in patients with heart failure and healthy controls. J Cardiovasc Pharmacol 2005;45:197-203.
- Barber MN, Gaspari TA, Kairuz EM, Dusting GJ, Woods RL. Atrial natriuretic peptide preserves endothelial function during intimal hyperplasia. J Vasc Res 2005;42:101-10.
- Campbell DJ, Woodward M, Chalmers JP, Colman SA, Jenkins AJ, Kemp BE, et al. Perindopril-based blood pressure-lowering therapy reduces amino-terminal-pro-B-type natriuretic peptide in individuals with cerebrovascular disease. J Hypertens 2007;25:699-705.
- Germano CM, de Castro M, Crescencio JC, Gallo L Jr, Antunes-Rodrigues J, Moreira AC, et al. The interaction of plasma renin activity and plasma atrial natriuretic peptide in 21-hydroxylase deficiency patients. J Endocrinol Invest 2005;28:300-4.
- Ehrenreich H, Sinowatz F, Glover V. Natriuretic peptide interaction with [3H]isatin binding sites in rat brain. Brain Res 2005;1042:119-24.
- Tsukada T, Rankin JC, Takei Y. Involvement of drinking and intestinal sodium absorption in hyponatremic effect of atrial natriuretic peptide in seawater eels. Zoolog Sci 2005;22:77-85.
- Schubert ML. Gastric secretion. Curr Opin Gastroenterol 2003;19:519-25.
- You SY, Wu XZ, Liu ML. Effects of dachengqi decoction on gut hormones and intestinal movement after cholecystectomy. Zhongguo Zhong Xi Yi Jie He Za Zhi 1994;14:522-4. Chinese..
- Guo HS, Cui X, Cui YG, Kim SZ, Cho KW, Li ZL, et al. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscle of rat. Acta Pharmacol Sin 2003;24:1021-6.
- Singh G, Maguire JJ, Kuc RE, Skepper JN, Fidock M, Davenport AP. Characterization of the snake venom ligand [125I]-DNP binding to natriuretic peptide receptor-A in human artery and potent DNP mediated vasodilatation. Br J Pharmacol 2006;149:838-44.
- Lee S, Park SK, Kang KP, Kang SK, Kim SZ, Kim W. Relationship of plasma Dendroaspis natriuretic peptide-like immunoreactivity and echocardiographic parameters in chronic haemo-dialysis patients. Nephrology (Carlton) 2004;9:171-5.
- Piao FL, Park SH, Han JH, Cao C, Kim SZ, Kim SH. Dendroaspis natriuretic peptide and its functions in pig ovarian granulosa cells. Regul Pept 2004;118:193-8.
- Kim JH, Yang SH, Yu MY, Lee HK, Kim SY, Kim SH. Dendroaspis natriuretic peptide system and its paracrine function in rat colon. Regul Pept 2004;120:93-8.
- Kim SZ, Kim SH, Park JK, Koh GY, Cho KW. Presence and biological activity of C-type natriuretic peptide-dependent guanylate cyclase-coupled receptor in the penile corpus cavernosum. J Urol 1998;159:1741-6.
- Jin JY, Li ZH, Li ZJ, Jin ZY, Jin NG, Li Y, et al. Effect of nitric oxide on electric and mechanical activities of gastric antral circular muscles in guinea pigs. Acta Pharmacol Sin 2000;21:369-73.
- Li Y, Xu WX, Li ZL. Effects of nitroprusside, 3-morpholino-sydnonimine, and spermine on calcium-sensitive potassium currents in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin 2000;21:571-6.
- Medvedev A, Crumeyrolle-Arias M, Cardona A, Sandler M, Glover V. Natriuretic peptide interaction with [3H]isatin binding sites in rat brain. Brain Res 2005;1042:119-24.
- Healy JM, Donald JA, Hyodo S, Toop T, Takei Y. Natriuretic peptide guanylyl cyclase receptors in the kidney of the Japanese eel, Anguilla japonica. Cell Tissue Res 2005;320:311-22.
- Itaba S, Chijiiwa Y, Matsuzaka H, Motomura Y, Nawata H. Presence of C-type natriuretic peptide (CNP) in guinea pig caecum: role and mechanisms of CNP in circular smooth muscle relaxation. Neurogastroenterol Motil 2004;16:375-82.
- Zahabi A, Picard S, Fortin N, Reudelhuber TL, Deschepper CF. Expression of constitutively active guanylate cyclase in cardio-myocytes inhibits the hypertrophic effects of isoproterenol and aortic constriction on mouse hearts. J Biol Chem 2003;278:47694-9.
- Ha KC, Piao CS, Chae HJ, Kim HR, Chae SW. Dendroaspis natriuretic peptide protects the post-ischemic myocardial injury. Regul Pept 2006;133:13-9.
- Ha KC, Chae HJ, Piao CS, Kim SH, Kim HR, Chae SW. Dendroaspis natriuretic peptide induces the apoptosis of cardiac muscle cells. Immunopharmacol Immunotoxicol 2005;27:33-51.
- Piao L, Li Y, Li L, Xu WX. Increment of calcium-activated and delayed rectifier potassium current by hyposmotic swelling in gastric antral circular myocytes of guinea pig. Acta Pharmacol Sin 2001;22:566-72.
- van der Zander K, Houben AJ, Kroon AA, De Mey JG, Smits PA, de Leeuw PW. Nitric oxide and potassium channels are involved in brain natriuretic peptide induced vasodilatation in man. J Hypertens 2002;20:493-9.