(5R)-5-hydroxytriptolide inhibits IFN-γ-related signaling1
Introduction
IFN-γ is a Th1-type cytokine and is principally generated by T lymphocytes. Two pathways participate in IFN-γ expression signaling: one is the IFN-γ-directed signaling in which IFN-γ promotes signal transducer and activator of transcription1 (STAT1) expression and activation, further enhances T-box transcription factor (T-bet expression), leading to the IFN-γ expression. The other is the IL-12-directed signaling, in which IL-12 induces STAT4 expression and activation, serving to amplify IFN-γ production[1]. Interferon regulatory factor-1 (IRF-1) is a transcription factor induced by IFN-γ and regulates interferon-related genes. IFN-γ tightly regulates the expression of various chemokines, including macrophage inflammatory protein (Mip)-1α, Mip-1β, regulated upon activation normally T-cell expressed and secreted (RANTES), inducible protein-10 (IP-10), monokine induced by IFN-γ (Mig), and IFN-inducible T cell a chemoattractant (I-TAC)[2]. The inhibition of these chemokines? expression will be of therapeutic potential, especially in rheumatoid arthritis.
There are 3 major groups of mitogen-activated protein (MAP) kinases in mammalian cells: the extracellular signal-regulated protein kinases (Erk), c-Jun N-terminal kinases (JNK)/stress-activated protein kinase (SAPK), and p38 mitogen-activated protein kinase (p38) MAP kinases[3,4]. Erk is normally associated with proliferation and growth factor induction. JNK and p38 are important for Th1 differentiation[5]. Both JNK and p38 MAP kinases are required for IFN-γ production by CD4+ Th1 cells[6].
(5R)-5-hydroxytriptolide (LLDT-8) is a novel triptolide derivate with potent immunosuppressive activities in vivo. LLDT-8 prevented graft-versus-host disease and prolonged cardiac allograft survival[7,8]. LLDT-8 inhibited type II collagen-induced arthritis[9] and suppressed concanavalin A (ConA)-induced liver injury[10]. Although LLDT-8 was demonstrated to inhibit the IFN-γ signaling in collagen-induced arthritis mice[9], the immune responses in vivo were too complex to clarify the precise action of LLDT-8. To better understand the underlying mechanisms of LLDT-8 action, in this study, we analyzed the effect of LLDT-8 on IFN-γ expression signaling in isolated murine T lymphocytes and on IFN-γ effector signaling in the macrophage cell line.
Materials and methods
Mice Male C57BL/6 mice and female BALB/c mice (6‒8 weeks old, 20‒22 g) were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China; Certificate N
Preparation of LLDT-8 LLDT-8 (C20H24O7; Mr=376; 99% purity) was synthesized from triptolide that was separated from the Chinese traditional herb Tripterygium wilfordii Hook F. The stock solution of LLDT-8 was prepared in dimethyl-sulphoxide (DMSO) and further diluted with pathogen-free saline (for in vivo study) or RPMI-1640 culture medium (for in vitro study) supplemented with 10% fetal bovine serum (FBS), 100 kU/L penicillin and 100 mg/L streptomycin.
Preparation of splenocyte Mice were sacrificed and their spleens were removed aseptically. A single cell suspension was prepared and cell debris and clumps were removed. Erythrocytes were lysed with Tris-buffered ammonium chloride. Mononuclear cells were washed and resuspended in culture medium[11]. A B cell-depleted population was prepared by immunomagnetic negative selection[12]. Briefly, cells were incubated with magnetic particles bound to goat anti-mouse Ig (Qiagen, Valencia, CA, USA), followed by removing cell-bound magnetic particles with a rare earth magnet (Polysciences Inc, Warrington, PA, USA). The purity of the T cells was analyzed with flow cytometry on a FACSCalibur (Becton Dickinson, San Jose, CA, USA), and was consistently >90%.
Flow cytometry analysis of T cell activation marker expression Anti-CD3 mAb (5 mg/L) were immobilized to a 24-well plate and incubated for 2 h at 37 °C. The wells were washed twice with PBS, and B cell-depleted spleen cells (3×109/L) from C57BL/6 mice were added in the presence of 100 nmol/L LLDT-8 for 24 h or 48 h. Cells were stained with fluorescein isothiocyanate (FITC) conjugated anti-mouse CD25 (a chain) or CD69 mAbs, or phycoerythrin (PE) conjugated anti-mouse CD154 mAb (BD Biosciences PharMingen, San Diego, CA, USA), and analyzed with flow cytometry.
Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled T cell division assay B cell-depleted spleen cells from C57BL/6 mice were washed with PBS and suspended in PBS at 1?1010/L. CFSE (Molecular Probes, Eugene, OR, USA) was then added to the cell suspensions at a final concentration of 1 mmol/L. The cells were incubated at 37 °C for 10 min and washed 3 times with culture medium. The resulting CFSE-labeled cells (3×109/L) were stimulated with ConA (5 mg/L) in the absence or presence of 100 nmol/L LLDT-8 for 48 h. Then the cells were harvested and stained with PE-conjugated anti-mouse CD4 or CD8 mAbs (PharMingen, USA), followed by flow cytometry analysis.
IFN-γ production B cell-depleted spleen cells (3×109/L) from C57BL/6 mice were cultured with 100 nmol/L LLDT-8 for 48 h in the anti-CD3 (5 mg/L)-coated plates. Culture supernatants were assayed for mouse IFN-γ by ELISA following the manufacturer’s instructions (PharMingen, USA).
Ovalbumin (OVA) immunization assay Female BALB/c mice were sc injected with 100 μg chicken egg OVA (Sigma, USA) dissolved in PBS and emulsified with an equal volume of Freund’s complete adjuvant containing the Mycobacterium tuberculosis strain H37Rv (Wako Pure Chemical Industries Ltd, Osaka, Japan). The mice were intraperitoneally injected with vehicle or 1 mg/kg LLDT-8 for 7 d.
Seven days later, B cell-depleted lymph node cells were prepared and cultured (4×109/L) with OVA (100 mg/L) for 48 h. Supernatants were collected for IFN-γ assay. When cultured for 72 h, the cells were pulsed with 0.25 μCi [3H]-thymidine for the last 8 h and harvested onto glass fiber filters. The incorporated radioactivity was counted using a Beta Scintillation Counter (MicroBeta Trilux, PerkinElmer Life Sciences, Boston, MA, USA).
RT-PCR and real-time PCR analysis B cell-depleted spleen cells (3×109/L) from C57BL/6 mice were cultured with 100 nmol/L LLDT-8 for 20 h in the anti-CD3 (5 mg/L)-coated plates. Cells were lysed using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was isolated, reverse transcribed, and PCR amplified using specific primers. RT-PCR products were visualized by electrophoresis through 1% agarose gels containing ethidium bromide. Relative quantitation with real-time PCR was performed with (it seems no spell-out for SYBR) Green PCR Reagents (Qiagen, USA) and a continuous fluorescence detection system (MJ Research, Waltham, MA, USA) were used according to the manufacturer’s instructions. The mRNA levels were normalized to those of hypoxanthine-guanine phosphoribosyl-transferase (HPRT).
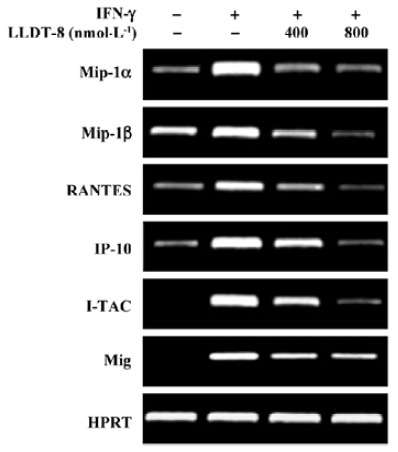
Raw 264.7 cells were pretreated with LLDT-8 (400 and 800 nmol/L) for 2 h before stimulation with 50 kU/L mIFN-γ for 6 h, followed by RT-PCR analysis. LLDT-8 at 800 nmol/L did not affect cell viability[13].
Western immunoblotting B cell-depleted spleen cells (5×109/L) from C57BL/6 mice were pretreated with 100 nmol/L LLDT-8 for 2 h before stimulation with anti-CD3 (5 mg/L) plus anti-CD28 (2 mg/L) for 30 min. Cells were lysed in SDS sample buffer (62.5 mmol/L Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 5% 2-mercaptoethanol and 0.02% bromophenol blue) and boiled for 5 min at 100 °C. Aliquots were electrophoresed in a 12% polyacrylamide gel and transferred to nitrocellulose membranes (Amersham Biosciences, Bucking-hamshire, UK). The membranes were treated with 10% non-fat milk for 1 h to block nonspecific binding, rinsed, and (cell signaling Technology) incubated with a panel of rabbit polyclonal Abs against total Erk1/2, phospho-Erk1/2, phospho-JNK/SAPK, phospho-p38 overnight at 4 °C. The membranes were then treated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG for 1 h. Immune complexes were detected with a chemiluminescence substrate (Pierce, Rockford, IL, USA) and exposed to Kodak X-ray film (Kodak, Rochester, NY, USA).
Statistical analysis Data are presented as mean±SEM or mean±SD where indicated. Student’s t-test and ANOVA were used to determine significance between the 2 groups where appropriate. P<0.05 was considered significant.
Results
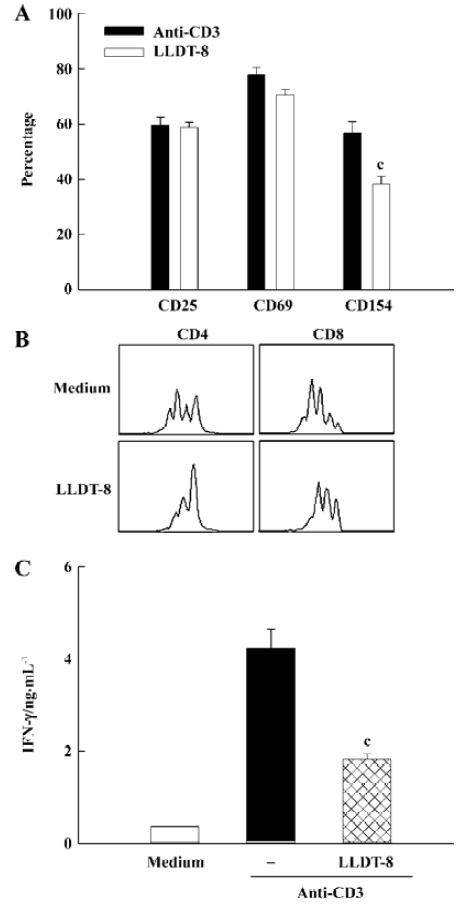
Effect of LLDT-8 on murine T lymphocyte immune responses in vitro To assess the effect of LLDT-8 on T cell activation, B cell-depleted murine splenocytes were stimulated with anti-CD3 and analyzed with flow cytometry. The expression levels of CD25, CD69, and CD154 indicated the activation state of T cells. Compared with the control culture, LLDT-8 at 100 nmol/L reduced CD154 expression by 30%, but did not affect CD25 and CD69 expressions in activated T cells (Figure 1A). Spleen cells were labeled with CFSE and activated with ConA for 48 h to assess cell division of T cell subsets. Data were presented as Figure 1B. Both CD4 and CD8 T cells underwent cell division after the ConA stimulation, which was markedly abrogated by 100 nmol/L LLDT-8. Moreover, LLDT-8 at 100 nmol/L suppressed IFN-γ production with statistical significance in anti-CD3-stimulated T cells (P<0.01; Figure 1C).
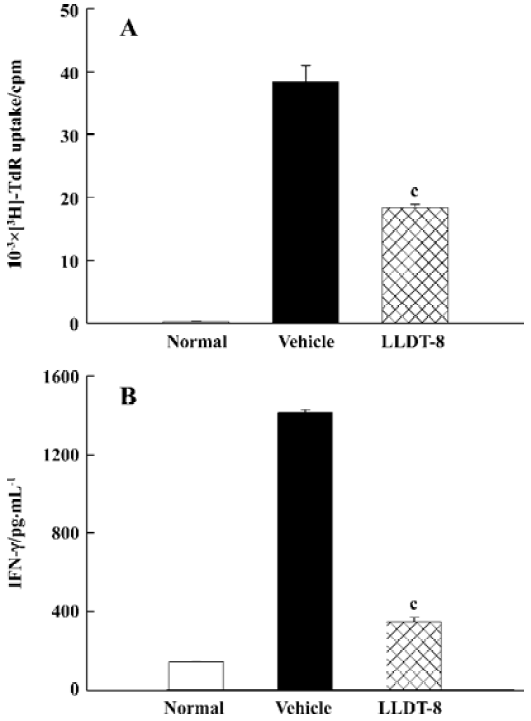
Treatment with LLDT-8 suppressed OVA-specific immune responses To test the capacity of antigen-specific T cell proliferation and IFN-γ production after in vivo LLDT-8 treatment, the mice were immunized with OVA and administered with LLDT-8 at 1 mg/kg. The proliferation of lymph node (LN) cells was examined. The results in Figure 2A show that the LN cells from the vehicle-treated mice proliferated well in response to OVA ex vivo. LLDT-8 treatment greatly impaired the OVA-specific proliferative response (P<0.01). We investigated the capacity of LN cells to produce IFN-γ with OVA stimulation. B cell-depleted LN cells produced large amounts of IFN-γ in the mice treated with vehicle alone, whereas the increased production of IFN-γ was reduced in the LLDT-8-treated mice (Figure 2B).
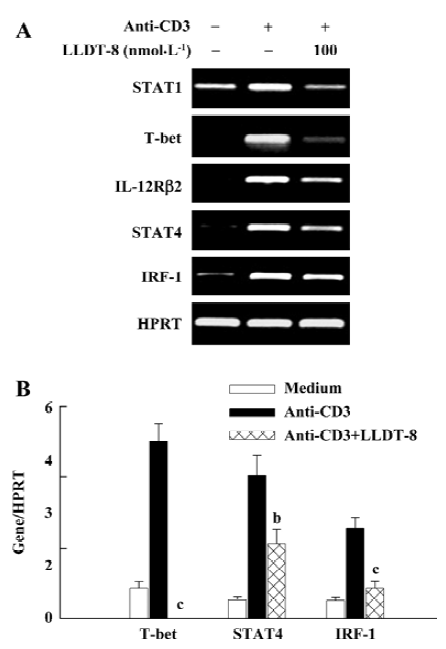
Inhibition of IFN-γ production signaling pathway by LLDT-8 treatment in vitro We further elucidated the reduction of IFN-γ by LLDT-8 at the mRNA level. Data are summarized in Figure 3. The transcription for STAT1, T-bet, IL-12 receptor β2 (IL-12Rβ2), STAT4 and IRF-1 increased greatly in anti-CD3-activated T cells. In the presence of 100 nmol/L LLDT-8, their mRNA expressions were retarded (Figure 3A). The T-bet, STAT4, and IRF-1 expressions were further analyzed with real-time PCR. Similar inhibitory activity of LLDT-8 was observed (Figure 3B).
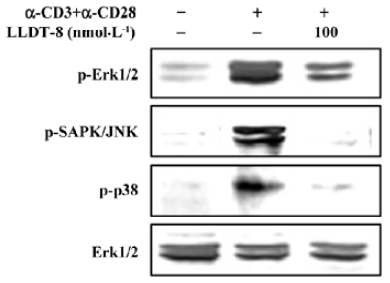
Effect of LLDT-8 on phosphorylation of Erk1/2, SAPK/JNK and p38 in T cells In mammalian species, MAP kinases are involved in all aspects of immune responses, from the initiation phase of innate immunity to the activation of adaptive immunity[5]. To better understand the inhibitory activity of LLDT-8, the activation of mitogen-activated protein kinase (MAPK) was analyzed. Anti-CD3 plus anti-CD28 effectively induced the phosphorylation of Erk1/2, SAPK/JNK and p38 (Figure 4). We examined the effect of LLDT-8 on their phosphorylation levels at 30 min after stimulation using Western immunoblot analysis. Treatment with 100 nmol/L LLDT-8 decreased the phosphorylation of these 3 kinases, displaying the strongest inhibition on p-SAPK/JNK (Figure 4).
LLDT-8 suppressed IFN-γ-triggered chemokine expression To evaluate the effect of LLDT-8 on IFN-γ-triggered signaling, expressions of a variety of chemokines were analyzed with RT-PCR at 6 h in IFN-γ-stimulated Raw 264.7 cells. Treatment with IFN-γ caused a dramatic increase in the transcripts of Mip-1α, Mip-1β, RANTES, IP-10, I-TAC, and Mig, and this increase was effectively abrogated by LLDT-8 (400 and 800 nmol/L; Figure 5).
Discussion
In our previous studies, LLDT-8 inhibited inflammatory and autoimmune diseases via suppressing T cell immune responses[7,9]. Some of those beneficial effects of LLDT-8 seemed to be associated with the reduction of IFN-γ production. In this study, the effect of LLDT-8 on IFN-γ signaling was investigated.
LLDT-8 did not prevent T cells from activation. The expression of activation markers CD25 and CD69 was not affected by LLDT-8. The CD154 (CD40 ligand) expression was only slightly reduced. Therefore, the inhibition of T cell immunity by LLDT-8 might occur after the T cells were activated. LLDT-8 effectively prevented T cell division. Importantly, it seemed that the inhibitory effect of LLDT-8 on CD4+ T cells was stronger than that on CD8+ T cells. LLDT-8 suppressed the TCR-triggered IFN-γ production; the inhibitory activity of LLDT-8 in T cell proliferation and IFN-γ generation was further demonstrated in the OVA-immunized mice.
The IFN-γ/STAT1/T-bet/IFN-γ pathway and the IL-12/IL-12Rβ2/STAT4/IFN-γ pathway mediate IFN-γ production[1]. LLDT-8 impaired the mRNA expressions of STAT1, T-bet, IL-12Rβ2 and STAT4, leading to the reduction of IFN-γ generation. IRF-1 is a transcription factor induced by IFN-γ. In the presence of LLDT-8, the IRF-1 expression was sup-pressed. In mammalian species, MAP kinases are involved in all aspects of immune responses[5]. Erk activation is an important event of T cell activation. LLDT-8 reduced Erk1/2 phosphorylation, indicating its role in T cell function. JNK and p38 MAP kinases have been implicated participating in inflammation[4]. JNK2, 1 member of the JNK family, is critical for the initial production of IFN-γ during the differentiation of Th1 cells[14]. p38 MAP kinase pathway is crucial for T cell-mediated immunity and the development of Th1 responses. Activation of the p38 pathway is required for IFN-γ gene transcription[5,15,16]. Moreover, persistent activation of p38 MAP kinase resulted in increased IFN-γ production by Th1 effector cells[17]. The phosphorylation levels of SAPK/JNK and p38 were significantly reduced by LLDT-8. This result indicated that LLDT-8 inhibited IFN-γ production and Th1 immune responses at least partially via preventing JNK/SAPK and p38 activation.
IFN-γ can regulate leukocyte trafficking through the induction of various chemokine genes, including Mip-1α, Mip-1β, RANTES, IP-10, Mig, and I-TAC[2]. IFN-γ induces Mip-1α, Mip-1β, IP-10, and Mig in the presence of STAT1[18]. IRF-1 response elements have been identified in the promoters of Mig, IP-10, and I-TAC[19,20]. Thus, IFN-γ may induce these chemokine expressions also via the IFN-γ/STAT1/IRF-1 pathway. LLDT-8 effectively inhibited the transcription of these chemokines in IFN-γ-stimulated Raw 264.7 cells, implicating the inhibitory effect on IFN-γ-triggered immune responses.
In summary, LLDT-8 inhibited IFN-γ production via the blockade of IFN-γ/STAT1/T-bet/IFN-γ signaling and IL-12Rβ2/STAT4/IFN-γ signaling. LLDT-8 suppressed the IFN-γ-induced transcription of IRF-1 and chemokines. The blockade of MAPK activation at least partially contributed to the suppressive effect of LLDT-8 on IFN-γ. Taken together, LLDT-8 was a promising IFN-γ inhibitor by targeting its production pathway and downstream effector pathway.
References
- Grogan JL, Locksley RM. T helper cell differentiation: on again, off again. Curr Opin Immunol 2002;14:366-72.
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004;75:163-89.
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410:37-40.
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK) — from inflammation to development. Curr Opin Cell Biol 1998;10:205-19.
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 2002;20:55-72.
- Rincon M, Flavell RA, Davis RA. The JNK and p38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med 2000;28:1328-37.
- Tang W, Yang Y, Zhang F, Li YC, Zhou R, Wang JX, . Prevention of graft-versus-host disease by a novel immunosup-pressant, (5)-5-hydroxytriptolide (LLDT-8), through expansion of regulatory T cells. Int Immunopharmacol 2005; 5: 1904-13.
- Tang W, Zhou R, Yang Y, Li YC, Yang YF, Zuo JP. Suppression of (5R)-5-hydroxytriptolide (LLDT-8) on allograft rejection in full MHC-mismatched mouse cardiac transplantation. Transplantation 2006;81:927-33.
- Zhou R, Tang W, Ren YX, He PL, Zhang F, Shi LP, et al. (5R)-5-hydroxytriptolide (LLDT-8) attenuated collagen-induced arthritis in DBA/1 mice via suppressing IFN-γ production and its related signaling. J Pharmacol Exp Ther 2006;318:35-44.
- Zhou R, Tang W, Ren YX, He PL, Yang YF, Li YC, et al. Preventive effects of (5R)-5-hydroxytriptolide on concanavalin A-induced hepatitis. Eur J Pharmacol 2006;537:181-9.
- Zhou R, Zhang F, He PL, Zhou WL, Wu QL, Xu JY, . ()-5-hydroxytriptolide (LLDT-8), a novel triptolide analog mediates immunosuppressive effects and . Int Immuno-pharmacol 2005; 5: 1895-903.
- Yang YF, Mukai T, Gao P, Yamaguchi N, Ono S, Iwaki H, et al. A non-peptide CCR5 antagonist inhibits collagen-induced arthritis by modulating T cell migration without affecting anti-collagen T cell responses. Eur J Immunol 2002;32:2124-32.
- Zhou R, Zheng SX, Tang W, He PL, Li XY, Yang YF, et al. Inhibition of inducible nitric-oxide synthase expression by (5R)-5-hydroxytriptolide (LLDT-8) in interferon-{gamma} and bacterial lipopolysaccharide stimulated macrophages. J Pharmacol Exp Ther 2006;316:121-8.
- Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ, Rincon M, et al. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity 1998;9:575-85.
- Dong C, Davis RJ, Flavell RA. Signaling by the JNK group of MAP kinases. c-jun N-terminal kinase. J Clin Immunol 2001;21:253-7.
- Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, . Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J 1999; 18: 1845-57.
- Rincon M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, et al. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J 1998;17:2817-29.
- Gil MP, Bohn E. Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci USA 2001;98:6680-5.
- Hamilton NH, Banyer JL, Hapel AJ, Mahalingam S, Ramsay AJ, Ramshaw IA, et al. IFN-gamma regulates murine interferon-inducible T cell alpha chemokine (I-TAC) expression in dendritic cell lines and during experimental autoimmune encephalomyelitis (EAE). Scand J Immunol 2002;55:171-7.
- Nazar AS, Cheng G, Shin HS, Brothers PN, Dhib-Jalbut S, Shin ML, et al. Induction of IP-10 chemokine promoter by measles virus: comparison with interferon-gamma shows the use of the same response element but with differential DNA-protein binding profiles. J Neuroimmunol 1997;77:116-27.
