Diallyl disulfide suppresses growth of HL-60 cell through increasing histone acetylation and p21WAF1 expression in vivo and in vitro1
Introduction
The development of agents that induce differentiation and/or apoptosis is a promising avenue for the treatment of human acute myeloid leukemia HL-60 cells. Epidemiologic studies offer evidence that a high garlic consumption reduces the risk of colorectal and stomach cancers[1]. These observations are supported by in vivo experiments carried out in rodents, concluding that garlic extract or garlic powder intake reduces chemically-induced carcinogenesis in different organs (the skin and the mammary gland)[2–4]. Diallyl disulfide (DADS) is a naturally occurring organosulfur compound derived from crushed garlic, which is the predominant lipid-soluble sulfide in essential garlic oil[5]. Some studies have shown that the antiproliferative activity of DADS is believed to be due to its ability to induce differentiation and/or apoptosis and to arrest cells in the G0/G1 or G2/M phase[6–9] in A549 human lung cancer, T24 human bladder cancer, breast, colon cancer cells, etc[10–13]. DADS-induced apopto-sis in human leukemia HL-60 cells is triggered by the generation of hydrogen peroxide, activation of caspase-3, degradation of poly(ADP-ribose) polymerase (PARP), and fragmentation of DNA[14]. In addition to the antiproliferative activity in cell culture and tumor xenograft models, DADS is highly effective for the prevention of chemically-induced cancers in animal models. Wattenberg et al were the first to demonstrate that N-nitrosodiethylamine-induced neoplasia of the forestomach in female A/J mice was inhibited by 90% upon po DADS administration prior to the carcinogen challenge[15]. The intragastric intubation of DADS also prevented colon and renal neoplasia in the multi-organ carcinogenesis model in male F344 rats[16].
The acetylation of histones appears to be an important mechanism for the regulation of gene transcription[17–19]. The acetylation of specific lysine residues, which occurs within the N-terminal domain of core histones, is one of the mechanisms involved in the modification of the chromatin structure, and is generally correlated with transcriptional gene activity. Histone acetylase (HAT) and deacetylase (HDAC) are emerging as important components of protein complexes that affect the dynamics of chromatin folding during gene transcription. In normal cells, histone acetylation levels are thought to result from an equilibrium between competing HAT and HDAC[20,21]. Histone acetylation is often associated with activated transcription, and deacetylation correlates with transcriptional silencing. Recently, some studies have shown that DADS antiproliferation is related to the increase of histone acetylation. DADS antiproliferative effects in human colon tumor cell lines HT-29 and Caco-2[22,23], and in DS19 mouse erythroleukemic cells[24], were associated with a transient increase of histone acetylation; more-over, DADS could inhibit nuclear HDAC activity. We previously reported that DADS could significantly inhibit the growth of MGC803 cells in vitro and in vivo, by activating p38 MAPK pathways to induce G2/M arrest of cells[25,26]. DADS had a significant antiproliferative effect and could also induce differentiation in leukemia HL-60 cells[27–29]. The objective of the present work was to establish whether DADS suppresses proliferation and induces differentiation of HL-60 cells through increasing histone acetylation and the expression of p21WAF1 in vitro and in vivo.
Materials and methods
Reagents DADS (purity 80%, the remaining 20% being diallyl trisulfide and diallyl sulfide), purchased from Fluka Co (Milwaukee, Wisconsin, USA), was dissolved in Tween 80[28]at 8 g/L and stored at -20 °C. Sodium butyrate (SB), which was purchased from Sigma Co (St Louis, Montana, USA), was dissolved in PBS at 100 mmol/L and stored at -20 °C it was used as the positive control.
Animals The severe combined immunodeficiency (SCID) mice, eighteen 3–4-week-old male (11–12 g), purchased from the Experimental Center of the Chinese Academy of Science in Shanghai, were fed common mouse feeds with eggs and maintained under specific pathogen-free conditions in the animal laboratory of our Cancer Research Institute.
Cell culture HL-60 cells, obtained from the Cancer Research Institute, Xiangya Medical College, Center South University in China, were cultured in RPMI-1640 medium with 10% fetal calf serum at 37 °C in a 5% CO2 incubator.
Differentiation assays Nitroblue tetrazolium (NBT) reduction was performed by the previously described methods to measure the differentiation induction of the HL-60 cells[29].
Protein extraction Cultures of HL-60 cells in the logarithmic growth phase at a density of 2×105 cells per mL were cultured with 1.25 µg/mL DADS for 12, 24, 48, or 72 h at 37 °C. Cells cultured without DADS were harvested for comparison of histone acetylation levels. At the appropriate time point, the cells were harvested, washed with ice-cold PBS and suspended in 0.5 mL lysis buffer [10 mmol/L Tris-HCl (pH 7.6), 100 mmol/L NaCl, 1 mmol/L EDTA (pH 8.0) and 100 µg/mL PMSF] containing protease inhibitor aprotinin (1 µg/mL). Lysates were centrifuged at 10 000×g for 10 min, and extracts were quantified using a background-corrected absorbance (BCA) protein quantified kit (Pierce, Rockford, IL, USA).
Inoculation of SCID mice HL-60 5×106 cells in the logarithmic growth phase were injected into the right side of the peritoneal cavity per mouse, and the mice were monitored until the peritoneal neoplasms were detected (28 d after the inoculation of the HL-60 cells).
Treatment of SCID mice with DADS SCID mice bearing HL-60 peritoneal neoplasms were randomly divided into 3 groups (6 mice per group). The mice received an ip injection of vehicle alone (NS), 42 mg/kg DADS or 73 mg/kg SB 3 times per week, for a total treatment period of 21 d. Injection volumes were kept constant at 0.2 mL for each mouse. Tumor length and width and abdomen circumference were measured twice weekly by vernier calipers, and tumor volume was calculated using the following formula:
Tumor volume=length×(width)2 ×π/6
After 21 d of treatment, at which time the control animals (third and fifth) had large neoplasms which required the animals to be sacrified, all of the mice were killed 12 h after the final injection and the neoplasms and livers were removed. Tissues were flash-frozen in liquid nitrogen or fixed in formalin and embedded in paraffin. During the treatment period, the mice were weighed twice weekly and monitored for any overt signs of toxicity.
Cycle distribution analysis of HL-60 cells in SCID The cycle distribution of HL-60 cells in SCID was monitored by flow cytometry analysis as follows: 100 mg tumor tissues were cut into mono-cell suspension in ice-cold PBS. The suspension cells were enumerated by a counter, and 1×106 cells were pelleted by centrifugation. The supernatant was aspirated and the cells were resuspended in 1 mL of ice-cold ethanol (75%) and stored for 24 h at 4 °C. After the ethanol was removed, the cells were incubated with PBS containing RNase at 37 °C for 30 min and then stained for 30 min with propidium iodide. Sample data were collected using a Becton Dickinson FACS (Franklin Lakes, NJ, USA) and analyzed with Verity Winlist Software (Verity Software House, Topsham, ME, USA).
Tissue protein extraction Frozen tumor tissues 100 mg were ground under liquid nitrogen and washed in ice-cold PBS, the PBS was discarded and the tissues were suspended in 0.5 mL lysis buffer [10 mmol/L Tris-HCl (pH 7.6), 100 mmol/L NaCl, 1 mmol/L EDTA (pH 8.0) and 100 µg/mL PMSF] containing protease inhibitor aprotinin (1µg/mL). After centrifugation at 10 000×g for 10 min, the supernatant collected was analyzed for protein content using a BCA protein quantified kit (Pierce, Rockford, IL, USA).
Western blot analysis Total proteins (20–25 µg/point) were separated by 12% SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The blots were blocked in 5% nonfat milk in Tris buffered saline (TBS) containing 0.1% Tween 20 for 2 h at room temperature, and incubated for 2 h at room temperature with the 1:1000 dilution of the acetylated histones H3 and H4 rabbit polyclonal primary antibodies (Upstate Biotechnology, Lake Placid, NY, USA) and with the 1:400 anti-p21WAF1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The blots were washed for 3×5 min in TBS-T and then incubated with a 1:1000 dilution of peroxidase-conjugated secondary antibody for 1 h at room temperature. The blots were again washed for 3×10 min in TBS-T and then developed by an enhanced chemiluminescence plus (ECL Plus) kit (Amersham Biosciences, Buckinghamshire, England). Where indicated, the blots were stripped and reprobed with antibodies directed against β-actin (Sigma-Aldrich, St Louis, Montana, USA).
Statistical analysis Data were expressed as Mean±SD. Group t-test was used to compare data between every 2 groups. One-way analysis of variance (ANOVA) was used to determine statistically significant differences among multiple groups. P<0.05 was considered statistically significant.
Results
Differentiation induction by DADS in HL-60 cells Table 1 showed the NBT reduction ability of HL-60 cells treated with DADS increased in a time-dependent manner, compared with no treatment of HL-60 cells in vitro (P<0.05).

Full table
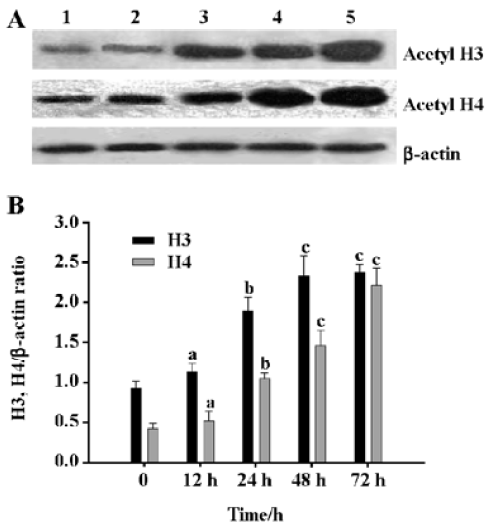
Induction of histone H4 and H3 acetylation in HL-60 cells by DADS DADS increased the acetylation levels of histones H4 and H3 in HL-60 cells. The effect of 1.25 µg/mL DADS on histone H4 and H3 acetylation was studied by Western blot. DADS induced histone H3 and H4 hyper-acetylation after 24 h incubation with significant 2.0-fold and 2.4-fold increase, respectively (Figure 1).
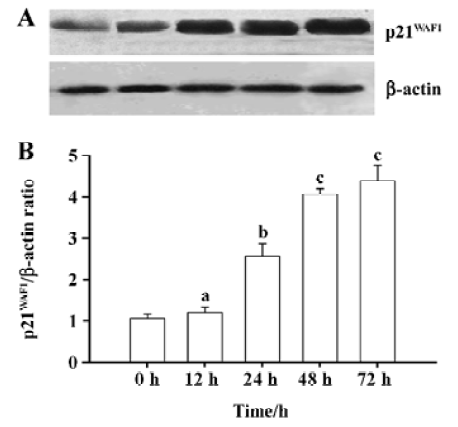
DADS increases p21WAF1 protein expression in HL-60 cells The p21WAF1 protein is an essential regulatory protein of cell cycle progression. Expression of its gene is partly regulated by histone acetylation. Since we observed that 1.25 µg/mL DADS induced histone hyperacetylation and increased HL-60 cell differentiation, we studied its effects on p21WAF1 protein levels. DADS 1.25 µg/mL induced significant increases in p21WAF1 protein levels after 24-h incubation (Figure 2).
Growth inhibition of the peritoneal neoplasm by DADS In order to evaluate the effects of DADS on HL-60 cell growth in vivo, we examined its antitumor efficacy using a SCID mouse model. After 21 d of treatment, the animals were killed and the tumor sizes were determined. The mean tumor volume in mice treated with NS was 5.47±0.57 cm3, the mean tumor volume in mice treated with DADS was 1.85±0.30 cm3, and that in the group treated with SB was 1.92±0.30 cm3. The difference in tumor size between the mice treated with DADS and those treated with SB did not achieve statistical significance (P>0.05), whereas the group treated with DADS showed markedly suppressed tumor growth compared with the NS control (P<0.01) (Figure 3).
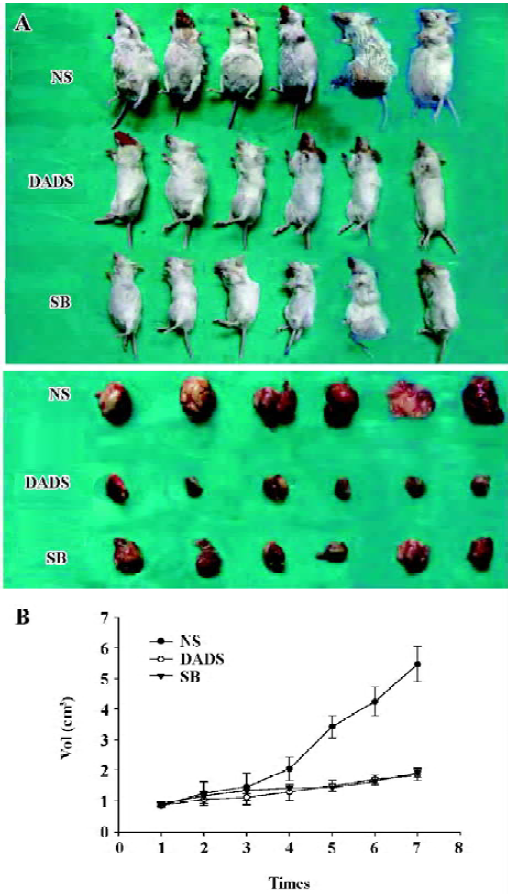
To monitor any possible toxicity arising from the treatment, the mice from all of the treatment groups were weighed twice weekly during the 21-d treatment period, No difference was detected among bundles and no mouse died throughout the treatment period.
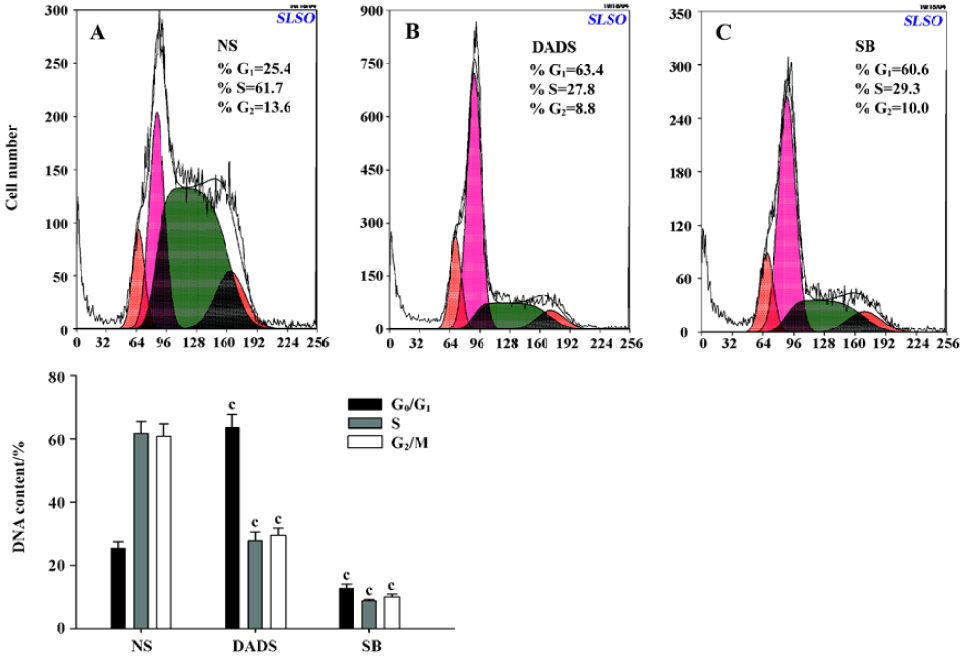
Cycle distribution of HL-60 cells in SCID The cycle distribution of HL-60 cells in SCID mice was monitored by flow cytometry analysis. The percentages of G0/G1 or S phase cells in the NS group were 25.4% and 61.7%, respectively. In contrast, the 42 mg/kg DADS and 73 mg/kg SB treatment led to a significant inhibition of DNA synthesis as evidenced by the fact that the percentages of G0/G1 phase cells increased to 63.4%; the S phase cells decreased to 27.8% in the DADS group (Figure 4).
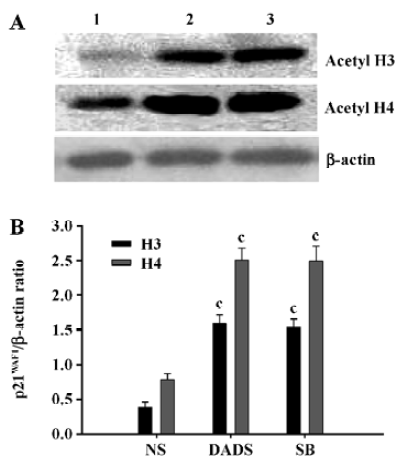
DADS increases acetylated histones in vivo Proteins were isolated from the excised tumors of 3 mice in each treatment group at the end of the 21-d treatment period. The tumors removed from the mice treated with 42 mg/kg DADS showed significant increase of acetylated histone H3 and H4, 4.1-fold and 3.2-fold increases, respectively, compared with the tumors from the NS group. There was no difference compared with the tumors of the mice treated with 73 mg/kg SB, an inhibitor of histone deacetylase (Figure 5).
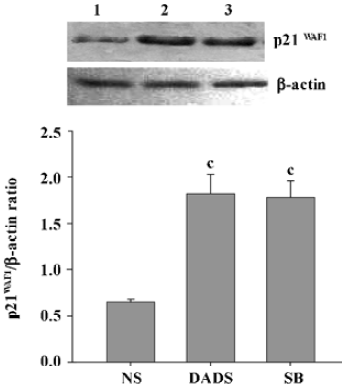
Increase of p21WAF1 expression in tumor cells by DADS Protein lysates were isolated from the sample removed from 3 mice in each group and analyzed for expression of the p21WAF1 protein. The tumors from the mice treated with DADS showed a 2.8-fold increase in the expression of the p21WAF1 protein, compared with tumors from the NS group; there was also a resemblance to the expression in the SB-treated group, an inhibitor of histone deacetylase (Figure 6).
Discussion
This study demonstrates that DADS is a potent inhibitor of HL-60 cell growth in vitro and in vivo. In vitro, our previous work showed that 1.25 µg/mL DADS inhibited HL-60 cell proliferation and induced differentiation. The cell surface differentiation antigen CD11b increased from 12% (untreated) to 44% (treated by 1.25 µg/mL DADS for 3 d) (P<0.01). G0/G1 cells were added to 44.6% (P<0.01), but S and G2/M phase cells descended to 48.5% and 6.9% (P<0.01), respectively (the ratio of G0/G1, S, and G2/M phase in untreated HL-60 cells were 23%, 66% and 11%, respectively), and the cell cycle was arrested at the G1 phase. Kinase activity of Janus kinase (JAK)/ signal transduction and activators of transcription (STAT) was tested by Western blot. The results demonstrated that the activity of phosphorylated Jak1 and Stat3 kinase was inhibited, the expression of Stat3 and c-myc gene decreased, c-fos and c-jun gene expression increased in HL-60 cells treated by DADS. The above results suggest that DADS could induce HL-60 cells differentiation toward granulocytic lineage, the inhibition of phosphorylated Jak1 and Stat3 was involved in HL-60 cell differentiation induced by DADS, and its possible molecular mechanism might relate to the modulation of proliferation-associated gene expression and inhibition of DNA synthesis[28–30]. In the present study, the NBT-reduction assay showed that the percentage of NBT-positive cells markedly increased after treatment with DADS, which suggests that DADS can induce differentiation of HL-60 cells. In vivo, the administration of DADS to SCID mice bearing HL-60 cells caused significant growth suppression of tumors at dose (42 mg/kg) with little detectable toxicity. Some studies have indicated that DADS inhibits the proliferation of human colon tumor cells[22,23], DS19 mouse erythroleukemia cells and K562 human leukemia cells[24] by increasing acetylated histone in vitro. In the present study, we found that DADS caused the increase of acetylated histone in HL-60 cells cultured with the agent within 24 h. The increase of acetylated histone was observed in the HL-60 cell xenografts after the administration of 42 mg/kg DADS by ip injection 3 times a week in SCID mice. The effects were similar to that of SB, which is a potent histone deacetylase inhibitors (HDI)[31]. Consequently, we presume that DADS may serve as a potential HDI and inhibits HL-60 cell growth. HDI have been reported to induce G1 or G2 phase arrest and regulate the transcription of a number of cell cycle regulator genes, including p21, c-myc, cyclin and cdk[32–34]. HDI are currently receiving considerable attention as antitumor agents because of their ability to induce cell cycle arrest and/or cell death in a wide range of transformed cells in vitro and in vivo[35,36]. Our previous work shows that DADS induced cell cycle arrest at the G0/G1 phase on HL-60 cells in vitro[29]. Here we found that DADS also induced cell cycle arrest at the G0/G1 phase on HL-60 cells in SCID mice in vivo. The cycle-dependent kinase inhibitor p21WAF1 has been identified as a target induced by HDI in transformed cells[37]. The expression of p21WAF1 in transformed cells after treatment with HDI was preceded by localized hyperacetylation of histones in the chromatin region containing the p21WAF1 gene[38,39]. These findings suggest that these agents act directly to induce hyperacetylation and thereby alter the chromatin structure in the region of the p21WAF1 gene. Our results show that DADS could induce hyperacetylation of histones and increase the expression of p21WAF1 in HL-60 cells. We then presume that DADS has an antitumor effect on HL-60 cells in vitro and in vivo because it could possibly cause hyper-acetylation of histones in the promoter region of the p21WAF1 gene. However, this requires further confirmation by chromatin immunoprecipitation assay.
In conclusion, DADS significantly inhibits the growth of human acute myeloid leukemia HL-60 cells in vitro and in vivo. The anticancer mechanism may involve the increase of histone acetylation, upregulation of p21WAF1 and G0/G1 phase arrest. DADS may be a potent antitumor agent for the management of human acute myeloid leukemia.
Acknowledgment
We would like to thank Lea MA (Department of Biochemistry and Molecular Biology, UMDNJ – New Jersey Medical School, Newark, NJ, USA) for criticism and encouragement.
References
- Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr 2000;72:1047-52.
- Sadhana AS, Rao AR, Kucheria K, Bijani V. Inhibitory action of garlic oil on the initiation of benzo(a)pyrene-induced skin carcinogenesis in mice. Cancer Lett 1988;40:193-7.
- Rao AR, Sadhana AS, Goel HC. Inhibition of skin tumors in DMBA-induced complete carcinogenesis system in mice by garlic (Allium sativum). Indian J Exp Biol 1990;28:405-8.
- Schaffer EM, Liu JZ, Green J, Dangler CA, Milner JA. Garlic and associated allyl sulfur components inhibit N-methyl-N-nitrosourea induced rat mammary carcinogenesis. Cancer Lett 1996;102:199-204.
- Milner JA. A historical perspective on garlic and cancer. J Nutr 2001;131:1027-31.
- Knowles LM, Milner JA. Possible mechanism by which allyl sulfides suppress neoplastic cell proliferation. J Nutr 2001;131:1061-6.
- Lan H, Lu YY. Allitridi induces apoptosis by affecting Bcl-2 expression and caspase-3 activity in human gastric cells. Acta Pharmacol Sin 2004;25:219-25.
- Park EK, Kwon KB, Park KI, Park BH, Jhee EC I. Role of Ca2+ in diallyl disulfide-induced apoptotic cell death of HCT-15 cells. Exp Mol Med 2002;34:250-57.
- Knowles LM, Milner JA. Diallyl disulfide induces ERK phosphorylation and alters gene expression profiles in human colon tumor cells. J Nutr 2003;133:2901-6.
- Wu XJ, Kassie F, Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res 2005;579:115-24.
- Lu HF, Sue CC, Yu CS, Chen SC, Chen GW, Chung JG. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol 2004;42:1543-52.
- Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis 2001;22:891-7.
- Sundaram SG, Milner JA. Diallyl disulfide suppresses the growth of human colon tumor cell xenografts in athymic nude mice. J Nutr 1996;126:1355-61.
- Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS, et al. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem Pharmacol 2002;63:41-7.
- Wattenberg LW, Sparnins VL, Barany G. Inhibition of N-nitro-sodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res 1989;49:2689-92.
- Takahashi S, Hakoi K, Yada H, Hirose M, Ito N, Fukushima S. Enhancing effects of diallyl sulfide on hepatocarcinogenesis and inhibitory actions of the related diallyl disulfide on colon and renal carcinogenesis in rats. Carcinogenesis 1992;13:1513-8.
- Spencer VA, Davie JR. Role of covalent modifications of histones in regulating gene expression. Gene 1999;240:1-12.
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature 2000;403:41-5.
- Turner BM. Histone acetylation and an epigenetic code. Bioessays 2000;22:836-45.
- Felsenfeld G, Groudine M. Controlling the double helix. Nature 2003;421:448-53.
- Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell 2002;108:475-87.
- Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, et al. Diallyl disulfide (DADS) increases histone acetylation and p21waf1/cip1 expression in human colon tumor cell lines. Carcinogenesis 2004;25:1227-36.
- Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, et al. Repetitive treatments of colon HT-29 cells with diallyl disulfide induce aprolonged hyperacetylation of histone H3 K14. Ann N Y Acad Sci 2004;1030:612-21.
- Lea MA, Randolph VM, Patel M. Increased acetylation of histones induced by diallyl disulfide and structurally related molecules. Int J Oncol 1999;15:347-52.
- Xiang SL, Xiao XL, Ling H, Liao QJ, Zhou XT, Dong L, et al. Antitumor effect of diallyl disulfide on human gastric cancer MGC803 cells xenograft in nude mice. Ai Zheng 2005;24:940-4. Chinese..
- Yuan JP, Wang GH, Ling H, Su Q, Yang YH, Song Y, et al. Diallyl disulfide-induced G2/M arrest of human gastric cancer MGC803 cells involves activation of p38 MAP kinase pathways. World J Gastroenterol 2004;10:2731-4.
- Tan LM, Zhang MX, Luo HM, Zeng YZ, Li JM, Cui ZW, et al. The initiation of G2/M checkpoint by diallyl disulfide requires the activation of p38 MAP kinase in HL-60 cells. Zhonghua Xue Ye Xue Za Zhi 2004;25:273-6. Chinese..
- Wu MH, Tang L, Li LP, Huang WG, Su Q. Effect of Growth Inhibition and Differentiation of HL-60 cell Induced by Diallyl Disulfide. Zhonghua Xue Ye Xue Za Zhi 2004;25:300-2. Chinese..
- Wu MH, Su Q, Cheng AL, Tan H, Song Y. Experimental study of HL-60 cell differentiation induced by diallyl disulfide. Chin Pharmacol Bull 2003;19:319-22. Chinese..
- Wu MH, Huang WG, Tan H, He J, Su Q. Induction of differentiation by diallyl disulfide through inhibition of JAK1/STAT3 in human leukemia HL-60 cells. Chin Pharmacol Bull 2005;21:580-3. Chinese..
- Zheng X, Chang RL, Cui XX, Kelly KA, Shih WJ, Lin Y, et al. Synergistic effects of clinically achievable concentrations of 12-O-tetradecanoylphorbol-13-acetate in combination with all-trans retinoic acid, 1alpha, 25-dihydroxyvitamin D3, and sodium butyrate on differentiation in HL-60 cells. Oncol Res 2000;12:419-27.
- Sambucetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, Trogani N, Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem 1999; 274: 34 940–7.
- Siavoshian S, Blottiere HM, Cherbut C, Galmiche JP. Butyrate stimulates cyclin D and p21 and inhibits cyclin-dependent kinase 2 expression in HT-29 colonic epithelial cell. Biochem Biophys Res Commun 1997;232:169-72.
- Sandor V, Senderowicz A, Mertins S, Sackett D, Sausville E, Blagosklonny MV, et al. P21-dependent G1 arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer 2000;83:817-25.
- Li XX, Lu J, Zhao YM, Huang BQ. Function of c-Fos-like and c-Jun-like proteins on trichostatin A-induced G2/M arrest in Physarum polycephalum. Acta Biochim Biophys Sin (Shanghai) 2005;37:767-72.
- Boyle GM, Martyn AC, Parsons PG. Histone deacetylase inhibitors and malignant melanoma. Pigment Cell Res 2005;18:160-6.
- Rocchi P, Tonelli R, Camerin C, Purgato S, Fronza R, Bianucci F, et al. p21Waf1/Cip1 is a common target induced by short-chain fatty acid HDAC inhibitors (valproic acid, tributyrin and sodium butyrate) in neuroblastoma cells. Oncol Rep 2005;13:1139-44.
- Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA 2000;97:10014-9.
- Sambucetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, Trogani N, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem 1999;274:34940-7.
