Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by the accumulation of long-survival plasma cells in the bone marrow. The median survival from diagnosis among patients treated with conventional chemotherapy is 3–4 years. Introduction of high-dose chemotherapy with stem cell support has led to improved survival, but these patients eventually relapse from their disease and it remains an incurable disease with the currently available therapeutic modalities[1]. Clearly, more effective and less toxic treatment options are needed in the battle against MM.
Resveratrol (trans-3,4',5-trihydroxystilbene), a polyphenolic phytoalexin found in the skin of red grapes, various other fruits, and root extracts of the weed Polygonum cuspidatum, has recently attracted considerable interest because of its inhibitory activity on multiple cellular and molecular events associated with tumor development. Resveratrol has been shown to have potent inhibitory effects on the growth of leukemic cells, breast and colon cancer cells, cervical tumor cells and gastric adenocarcinoma cells[2–4]. In vivo, resveratrol inhibits three major steps of carcinogenesis: initiation, promotion, and progression[5]. Resveratrol has been considered as one of the most promising cancer chemopreventive or chemotherapeutic agents. However, the molecular mechanism by which resveratrol exerts its anticancer effect is largely unknown.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent neutral endopeptidases capable of degrading many components of the extracellular matrix (ECM) and basement membranes[6]. Recently, studies found that MMPs have a multifunctional role in the pathogenesis of MM. MMPs can contribute to cancer growth, invasion, angiogenesis, bone degradation and other processes important in the pathogenesis of MM. MMP inhibition not only resulted in reduction of tumor growth, but also had a significant effect on neovascularization and bone disease development[7]. Thus, the purpose of the present study was to examine if resveratrol affects MMPs and to evaluate the antitumor activity of resveratrol against MM cells.
Materials and methods
Materials Human MM cell lines RPMI 8226 and U266 were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The KM3 cell line was kindly provided by Prof Jian HOU (Senond Military Medical University, Shanghai, China). All the tumor cell lines were maintained in culture in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum in an atmosphere of 5% CO2 at 37 °C. Resveratrol, 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and gelatin were purchased from Sigma Chemical Co (St Louis, MO, USA). Rabbit anti-MMP-2, anti-MMP-9, anti-Bax, anti-Bcl-2, and anti-Bcl-xL polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti- X-linked inhibitor of apoptosis protein (XIAP) polyclonal antibodies were purchased from R&D systems (Minneapolis, MN, USA). Goat anti-rabbit horseradish peroxidase (HRP) conjugate was purchased from Zhongshan Company (Beijing, China). Chemiluminescence (ECL) reagent was obtained from Amersham Pharmacia Biotech (Piscataway, NJ, USA). A stock solution of resveratrol was made in dimethylsulfoxide (DMSO) at a concentration of 100 mmol/L and stored at -20 °C. RPMI-1640 and FBS were obtained from Gibco Life Technologies (Burlington, Ontario, Canada). Annexin V-PI Staining Kit (BMC306FI) was obtained from Bender Medsystems Inc (Burlingame, CA, USA). The transwell plate was obtained from Corning Costar (Cambridge, MA, USA). All other chemicals were purchased from authentic sources and were of the highest grade and purity.
MTT assay The effect of resveratrol on cell proliferation was measured using an MTT based assay. Briefly, the cells (5000/well) were incubated in triplicate in a 96-well plate in the presence of various concentrations of resveratrol (12.5, 25, 50, 100, 200 µmol/L) in a final volume of 0.2 mL for the indicated times. Thereafter, 0.025 mL of MTT solution (5 g/L) was added to each well and then incubated for 4 h. After centrifugation, the supernatant was removed from each well. The colored formazan crystal produced from MTT was dissolved in 0.15 mL of DMSO and then the optical density (OD) value was measured at 490 nm by a multiscanner autoreader (Dynatech MR 5000, Chantilly, VA). The following formula was used: percentage cell viability=(OD of the experimental samples/OD of the control) ×100.
Flow cytometric analysis To determine the apoptosis, resveratrol-treated cells were washed in PBS and resuspended in binding buffer at a concentration of 1×109 cells/L. After incubation, 195 µL of the solution was transferred to a 5 mL culture tube with 5 µL annexin V-FITC added. The tube was then incubated for 30 min at room temperature in the dark. The cells were washed with binding buffer and resuspended in 190 µL binding buffer, with 10 µL PI added. Finally, the tube was gently vortexed and incubated for another 30 min in the dark, and then the cells were analyzed immediately by flow cytometry.
Cell invasion Assay The inhibitory effect of resveratrol on VEGF-induced invasion was demonstrated in 24-well transwell cell culture chambers with the upper chamber containing filters of 8.0-µm pore size. The upper surface of the filters was coated with a mixture of basement membrane components (Matrigel matrix, 25 µg/filter) and dried overnight at 37 °C. RPMI 8226 cells were serum-starved in RPMI-1640 with 1% FBS for 12 h and then collected. After diluting cells in RPMI-1640 containing 1% FBS, 100 000 cells were seeded on the upper chamber wells together with or without resveratrol (12.5, 25, 50, 100 µmol/L). RPMI-1640 medium containing 1% FBS plus 25 µg/L VEGF was placed in the lower chamber as a chemotactant. After 24 h of incubation at 37 °C, cells that invaded into the lower compartment were counted by means of a Coulter counter ZBII (Coulter Electronics, Bedfordshire, England).
Gelatin Zymography Gelatin zymography was performed in KM3 conditioned media to visualize the gelatinolytic activity of secreted MMP-2 and -9. 1×106 cells/well were plated and treated with various concentrations of resveratrol (25, 50, 100, 200, and 400 µmol/L) in 6-well plates and incubated for 24 h at 37 °C. After incubation, the cells were washed with serum-free medium, replated in 0.5 mL serum-free medium and incubated for 24 h at 37 °C. The conditioned media were collected and centrifuged at 4000 rpm for 10 min to remove cell debris. A total of 10 µg of proteins from each conditioned medium were applied in duplicate to 10% SDS-PAGE gels copolymerized with type A gelatin at a final concentration of 0.1% under non-reducing conditions. After electrophoresis, gels were washed in 2.5% Triton X-100 for 1 h to remove SDS, incubated for 18 h at 37 °C, and stained in 0.1% Coomassie brilliant blue. The gelatinolytic regions were observed as white bands against a blue background. The levels of MMP activity were assessed by scoring the OD of the bands by a computerized image analysis Gel Pro 3.0 (Media Cybernetics, LP Silver Spring, MD).
Western blot analysis Conditioned medium (30 µL) was used for MMP-2 and -9 analysis. For the analysis of Bcl-2, Bcl-xL, Bax and XIAP, whole cell extracts were prepared by lysing the resveratrol-treated cells in lysis buffer (50 mmol/L Tris-HCl, pH 8.0; 150 mmol/L NaCl; 1 mmol/L EDTA, 1% Triton-X 100; 1 mg/mL aprotinin; 1mg/mL leupeptin and 100 µg /mL PMSF). Lysates were then spun at 14 000 rpm for 10 min to remove insoluble material. 30 to 60 µg protein extracts were separated by 10% SDS-PAGE under reducing conditions and then transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, membranes were incubated overnight at 4 °C with respective primary antibody. Levels of GAPDH or β-actin were confirmed to ensure equal loading of the samples. After washing, membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 1 h. Blots were then developed using ECL. Densitometric analysis of protein bands was performed using Gel Pro 3.0.
Statistical analysis Data were expressed as mean±SD. Statistical significance of difference observed in resveratrol-treated versus control cultures was determined by means of Student t-test. The minimal level of significance was P<0.05.
Results
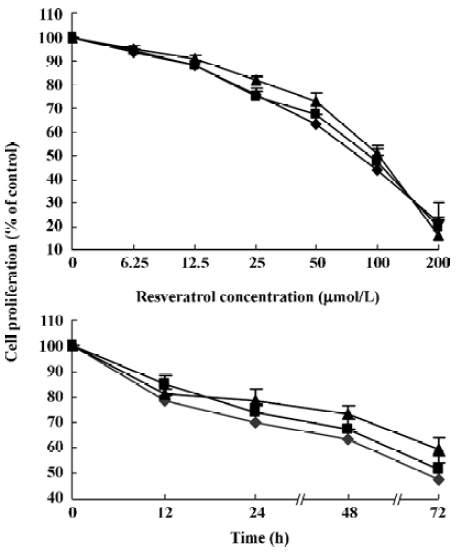
Resveratrol suppresses the proliferation of MM cells Resveratrol had significant growth inhibition effects on MM cells. MM cell lines RPMI 8226, U266, and KM3 were cultured in complete medium in the absence (control) or presence of various concentrations of resveratrol for indicated time periods. Untreated cells (control) were considered as the baseline (100%) for the analysis. Resveratrol inhibited cell growth of all 3 MM cell lines in a dose- and time- dependent manner (Figure 1A, 1B). The concentrations of resveratrol needed to inhibit cell growth of RPMI8226, U266, and KM3 were quite similar. Their IC50 values at 48 h were 72.3±9.6 µmol/L, 74.1±7.8 µmol/L, and 80.3±6.5 µmol/L, respec-tively.
Resveratrol induces apoptosis in MM cells To study whether resveratrol induced apoptosis in MM cells, we examined the expression of annexin V and exclusion of PI using two-color flow cytometry. Resveratrol induced apoptosis in RPMI 8226 cells in a dose-dependent fashion, the percentage of cells undergoing apoptotic cell death increased from 7.3% in the control culture to 36.9% after exposure to 100 µmol/L resveratrol for 24 h (Table 1). In the case of KM3 and U266 cell lines, similar results were obtained (data not shown).
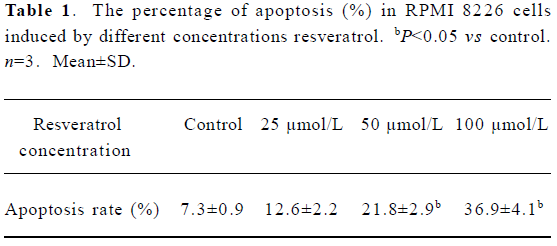
Full table
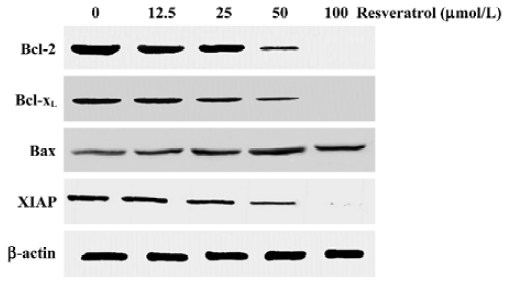
Resveratrol down-regulates Bcl-2, Bcl-xL, and XIAP and up-regulates Bax protein levels Analysis of the expression of the proteins involved in the apoptotic pathway revealed that resveratrol reduced the expression of the antiapoptotic proteins Bcl-2, Bcl-xL, and XIAP and induced the expression of the proapoptotic protein Bax (Figure 2). Resveratrol reduced the levels of Bcl-2, Bcl-xL, and XIAP proteins in a dose-depedent manner. A complete decline can be seen at 100 µmol/L resveratrol treatment. Likewise, resveratrol up-regulated Bax protein level in a dose-dependent manner. The expression of Bax was up-regulated at 12.5 µmol/L treatment and the effect was even more evident at 50 µmol/L.
Resveratrol inhibits MMPs in MM cells MM cell line KM3 was shown to constitutively release MMP-2 and MMP-9 with gelatinolytic activities at 62 kDa and 88 kDa, respectively, indicating that both enzymes are present in their cleaved, activated form. The levels of MMP-2 were much higher than those of MMP-9. 24 h treatment of KM3 cells with resveratrol reduced the gelatinolytic activity of MMP-2 as well as of MMP-9 in a concentration-dependent manner. The effect of resveratrol on MMP-9 appeared more dramatic than its effect on MMP-2 (Figure 3A).
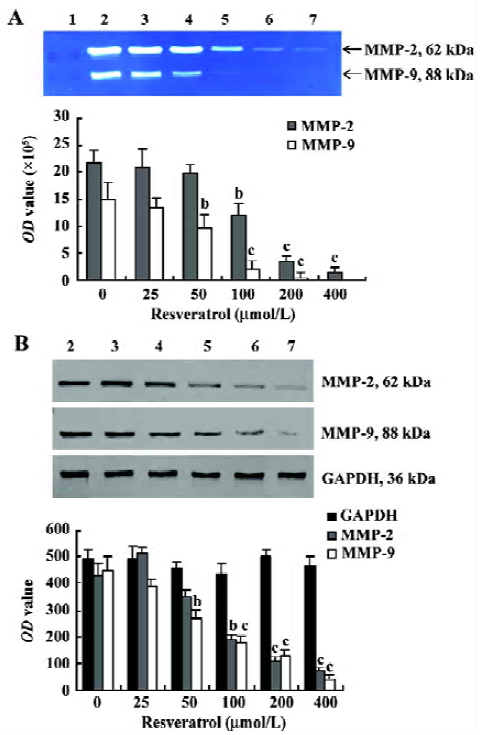
To investigate whether the resveratrol-mediated inhibition of MMP-2 and MMP-9 gelatinolytic activities could be attributable to the inhibition of the release of MMP-2 and MMP-9 protein, the amount of MMP-2 and MMP-9 proteins released by KM3 cells was further assessed by Western blotting. As shown in Figure 3B, resveratrol treatment markedly reduced the amount of MMP-2 and MMP-9 protein in the conditioned medium.
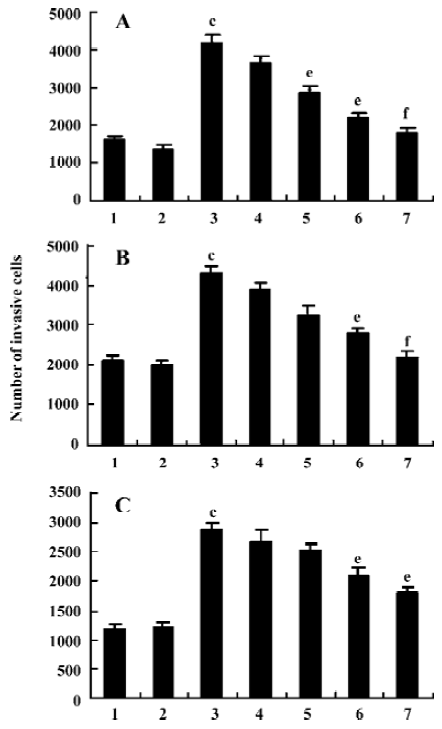
Resveratrol inhibits invasion of MM cells The effect of resveratrol on cell invasion was tested using Matrigel matrix-coated Boyden chambers. The addition of VEGF (25 µg/L) to the lower chamber induced transmigration of RPMI 8226, U266, and KM3 cells through the Matrigel (Figure 4A–4C). The VEGF-induced response was inhibited by resveratrol in a concentration-dependent manner with an average IC50 value of 64±8 µmol/L, 93±11 µmol/L, and 153±11 µmol/L, respectively.
Discussion
Resveratrol is a naturally occurring phytoalexin and a polyphenolic compound. The molecule was found to be present in various fruits and vegetables and is abundant in grapes and red wine. Recently, resveratrol has been the focus of numerous research investigations due to its anti-oxidative, anti-inflammatory, estrogenic effects as well as chemopreventive and antitumor activities[5,8,9]. Resveratrol acts on the process of carcinogenesis by affecting the three phases: tumor initiation, promotion and progression phases and suppresses the final steps of carcinogenesis, ie angiogenesis and metastasis. It is also able to activate apoptosis, to arrest the cell cycle or to inhibit kinase pathways. Interestingly, resveratrol does not present any cytotoxicity in animal models[4,10,11]. Recent studies in humans have revealed that resveratrol is pharmacologically quite safe[4,12]. Thus, the molecule has been considered a promising drug for cancer chemoprevention.
In the present study we showed that resveratrol inhibited the proliferation of all tested MM cells in a dose- and time- dependent manner, in agreement with similar findings recently reported by Jazirehi and Bonavida[13]. FACS analysis revealed that treatment of RPMI 8226 cells with resveratrol for 24 h increased annexin V-positive and PI-negative populations as well as annexin V and PI-positive populations in a dose-dependent fashion, suggesting that at least part of the resveratrol-induced suppression as observed in the present study is mediated through the induction of apoptosis. Resveratrol exerts its apoptotic effects by down-regulation of Bcl-2, Bcl-xL, and XIAP antiapoptotic proteins along with significant up-regulation of Bax proapoptotic protein.
Degradation of ECM is crucial for malignant tumor growth, invasion, metastasis, and angiogenesis. Matrix degradation is due to the secretion of a variety of enzymes, the most important belonging to the family of the MMPs. MMPs are a family of zinc-dependent neutral endopeptidases with proteolytic antivity for a large range of components of the ECM. Elevated levels of distinct MMPs can be detected in tumor tissue or serum of patients with advanced cancer, and their role as prognostic indicators in cancer has been widely examined[14]. In myeloma, it has been shown that tumor cells from patients with MM and from 5T33MM mice, bearing murine myeloma cells, secretes MMP-9 and that this secretion is induced by the interaction of MM cells with the BM microenvironment. BM stromal cells from patients with MM are also an important source of MMPs, mainly MMP-2[7]. Morever, secretion of MMPs by plasma cells parallel the progression of human MM[15]. In the present study, the KM3 expressed and secreted high levels of MMP-2, and sizable levels of MMP-9 were coexpressed and cosecreted. Our results are consistent with the previous studies that U266 cells predominantly secreted MMP-2 and to a less extent, MMP-9[16]. Resveratrol suppressed the gelatinolytic activity of MMP-2 as well as of MMP-9 in a concentration-dependent manner. As reported, MMPs family is involved in tumor progression, and inhibition of MMPs may suppress the invasion of tumor cells. We tested the effect of resveratrol on MM invasion. We found that treatment of MM cells with resveratrol abolished the VEGF-induced invasion. Our results corroborate those of Krishna et al, who found that inhibition of constitutive expression of MMP-9 protein by METVAN inhibited leukemic cell invasion through Matrigel matrix [17].
However, the concentrations of resveratrol that have been shown to elicit bioactivities in vitro are difficult to achieve in vivo in the intact mammalian target organ[18,19]. Results from preclinical studies in rats suggested that peak plasma levels of unmetabolized resveratrol are well below 10 µmol/L even after a high oral dose of 50 mg/kg, and its elimination is rather rapid. In contrast, resveratrol conjugates seem to reach much higher plasma levels than the parent agent[18]. Bioavailability studies in humans also demonstrated that the detected amounts of free resveratrol in plasma were very low (few µg/L or less) after moderate consumption of red wine or large amount of pure resveratrol consumption[19]. Therefore, the new routes of administration of resveratrol leading to high tissue concentrations in target organ should be explored and the potential biological activity of resveratrol metabolites should be considered for future investigations.
In conclusion, our results demonstrate that resveratrol is an effective inhibitor of MMPs in human MM cells. Resveratrol plays a role in suppressing the proliferation and invasion of MM cells and in inducing apoptosis. Validation of our in vitro findings with an in vivo model system is warranted for the potential clinical application in the management of patients with MM.
Acknowledgments
We thank Prof Jian HOU (Second Military Medical University, Shanghai) for providing the multiple myeloma cell line KM3 and Prof Tang-chun WU (Institute of Occupational Medicine, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan) for offering relevant experimental facilities and technical support.
References
- Anderson KC, Hamblin TJ, Traynor A. Management of multiple myeloma today. Semin Hematol 1999;36:3-8.
- Zoberi I, Bradbury CM, Curry HA, Bisht KS, Goswami PC, Roti Roti JL, et al. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett 2002;175:165-73.
- Bhat KP, Pezzuto JM. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res 2001;61:6137-44.
- Dorrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res 2001;61:4731-9.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997;275:218-20.
- Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 2004;48:411-24.
- Van Valckenborgh E, Croucher PI, De Raeve H, Carron C, De Leenheer E, Blacher S, et al. Multifunctional role of matrix metalloproteinases in multiple myeloma: a study in the 5T2MM mouse model. Am J Pathol 2004;165:869-78.
- Fremont L. Biological effects of resveratrol. Life Sci 2000;66:663-73.
- Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res 1999;25:65-77.
- Cao Y, Fu ZD, Wang F, Liu HY, Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J Asian Nat Prod Res 2005;7:205-13.
- Tseng SH, Lin SM, Chen JC, Su YH, Huang HY, Chen CK, et al. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin Cancer Res 2004;10:2190-202.
- Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 2001;22:1111-7.
- Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther 2004;3:71-84.
- Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets 2005;5:203-20.
- Vacca A, Ribatti D, Presta M, Minischetti M, Ria R, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 1999;93:3064-73.
- Vacca A, Ribatti D, Iuriaro M, Albini A, Minischetti M, Bussolino F, et al. Human lymphoblastoid cells produces extracellular matrix-degrading enzymes and induce endothelial cell proliferation, migration, morphogenesis, and angiogenesis. Int J Clin Lab Res 1998;28:55-68.
- Narla RK, Dong Y, Klis DT. TUckun FM. Bis(4,7-dimethyl-1,10-phenanthroline) sulfatooxovanadium(IV) as a novel antileukemic agent with matrix metalloproteinase inhibitory activity. Clin Cancer Res 2001;7:1094-101.
- Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther 2002;302:369-73.
- Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi P, et al. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res 2005;49:495-504.
