Influence of age and sex on pharmacodynamics of propofol in neurosurgical patients: model development1
Introduction
The dose requirement of many hypnotic and analgesic drugs used in anesthesia is reduced in elderly persons[1–2]. This can be explained by age-related changes in pharmaco-kinetics, pharmacodynamics, or both. Schuttler et al analyzed 4112 samples of blood from 270 individuals (150 men, 120 women, aged 2–88 years, weighing 12–100 kg) and concluded that the pharmacokinetics of propofol can be well described by a 3-compartment model. Inclusion of age and weight as covariates significantly improved the model[3]. Recently, we also showed that the pharmacokinetics of propofol changed with age and weight[4]. In children aged 1-5 years, a pharmacokinetic model for propofol was described using sparse data. In contrast to adults, body weight was a significant covariate for clearance in children[5]. The purpose of the present analysis was to establish a model for the pharmacodynamics of propofol in neurosurgical patients by examining cerebrospinal fluid (CSF) concentration, and to explore the covariates that influence the pharmacodynamic parameters of propofol. Specifically, we wanted to investigate the influence of age, sex, and weight on the pharmacodynamic characteristics of propofol. As in previous studies[6,7], we used the bispectral index (BIS) as a sensitive and continuous measure of drug hypnotic effect.
Materials and methods
After obtaining approval from the Human Investigation Committee of the First Affiliated Hospital, Zhejiang University College of Medicine and informed consent from the patients, 27 patients, American Society of Anesthesiologists physical status I and II, without symptoms of apparent increased intracranial pressure (ICP), who were scheduled for elective removal of intracranial tumors, were studied. Patients were excluded from the study if they had serious impairment of respiratory, cardiovascular, hepatic, renal, or endocrine function; or if they were receiving medication likely to influence the course of anesthesia.
Patient preparation Patients did not receive any premedication. Once the patient arrived in the operating room, a catheter was placed in the radial artery for continuous monitoring of arterial blood pressure. One intravenous cannula was inserted into a large forearm vein for infusion of propofol only, and another in the contralateral arm for infusion of fluid. As a part of the surgical procedure, an external drainage system (Codman; Johnson and Johnson Medical, Bracknell, UK) was inserted into the subarachnoid cisterns. Other variables monitored included the BIS index, end-tidal carbon dioxide partial pressure, heart rate, electrocardiogram parameters, and oxyhemoglobin saturation. One hundred percent oxygen (4 L/h) was provided through nasal prongs.
Drug administration and cerebrospinal fluid sampling After receiving crystalloid solution (Ringer's solution) at a dose of 20 mL/kg body weight, all patients were administered a bolus dose of propofol (Disoprivan or Diprivan; Zeneca Pharmaceuticals, Macclesfield, UK) at a dosage of 2 mg/kg for 5 min, followed by a constant infusion rate of 10 mg/kg per hour for 5 min by a manually controlled infusion pump (Graseby 3 500 pump; Graseby Medical, Watford, UK). When a patient suffered dyspnea, appropriate respiratory support was supplied. Hypotension was defined as a decrease in systolic blood pressure by more than 30% of the preanesthetic value or a systolic blood pressure <90 mmHg. Hypotension was treated by administering 6 mg iv ephedrine and crystalloid fluids. Bradycardia (<55 beats per min) was treated by administering 0.5 mg of iv atropine.
Cerebrospinal fluid samples of 2.5 mL for the determination of propofol concentration were obtained before infusion of propofol and during continuous infusion of propofol according to the BIS values. After the start of the infusion of propofol, we took one sample at each of the following BIS values: 100 –80, 80–70, 70–60, 60–50, and 50–30. The BIS was also recorded. Only CSF samples without red blood cells were saved for further analysis. Propofol concentrations in CSF were measured by high-pressure liquid chromatography with fluorescence detection[8]. After the evaluation at 30 min, the study was complete and the induction of anesthesia began.
Data analysis Demographics data are expressed as mean±SD. Statistical significance was defined for overall α error at the 0.05 level. The coefficient of correlation between predicted and measured CSF concentration of propofol were determined by linear regression. The following software packages were used to perform analyses: Excel 2000 (Microsoft, Redmond, VA, USA) and SPSS 11.0 (SPSS, Chicago, IL, USA) and NONMEM (nonlinear mixed-effect model; NONMEM Project Group, University of California, San Francisco, CA, USA)[9]. All the population analyses were performed using the Laplacian estimation method with the “likelihood” option as implemented in NONMEM. The results from the population analysis are presented as model estimate, together with the relative standard error, computed as the ratio between the standard error of the estimate and the value of the estimate.
Pharmacodynamics All data for the concentration of propofol in CSF and the corresponding BIS index were pretreated in Excel 2000. The correlations between the concentrations versus the original BIS value, the descending value from the baseline, and the percentage of the descending value from the baseline were performed by linear, exponential and polynomial regression. From the result of regression analysis (Table 1), we finally chose the original BIS value versus CSF concentration of propofol to perform the pharmacodynamic analysis.
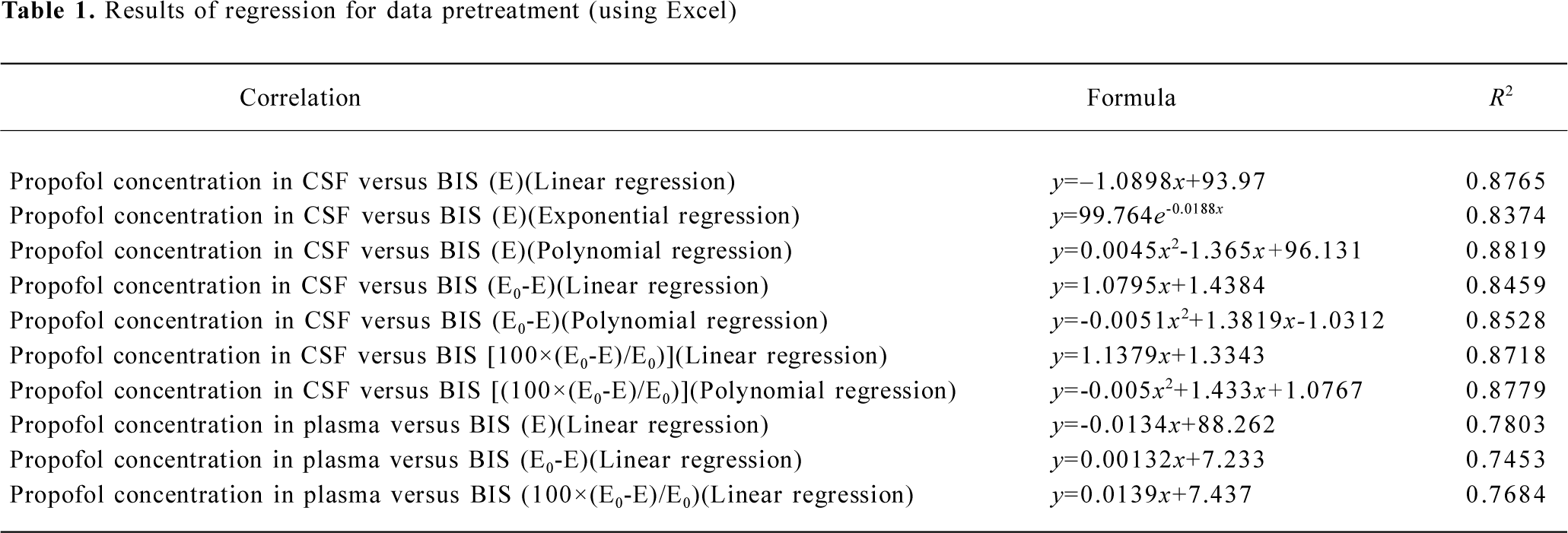
Full table
Model selection According to the previous data pretreatment, we firstly performed model analysis of linear, binomial, exponential and sigmoid Emax, respectively, for concentration-effect profiles without any covariates. Equations 1 to 4 demonstrate the abovementioned models.
where E is the effect of propofol, a,b and d are constants, and C is the concentration of propofol. Emax denotes the maximum effect of propofol; EC50 is the concentration of propofol associated with 50% of the maximum effect and N is the Hill coefficient.
Subsequently, we incorporated additional possible covariates of age, sex, and weight successively. For model selection, the minimum value of the objective function (OBJF) was considered together with the parameters of model, performance error (PE; %), median absolute performance error (MDPE; %), and the percent standard error of the mean (SEM; %). A model was considered superior to another nested model when the minimum value of the OBJF was reduced by 3.85 points. The difference in OBJF between two nested models is approximately χ2 distributed and can be used for significance tests (P<0.05, with one degree of freedom). In addition, when incorporated one of the factors of age, sex, and weight, the parameters of the model were optimized, error was not increased, then the factor is considered as a covariate of the propofol pharmacodynamic model. Tables 2 and 3 show the models we have developed.
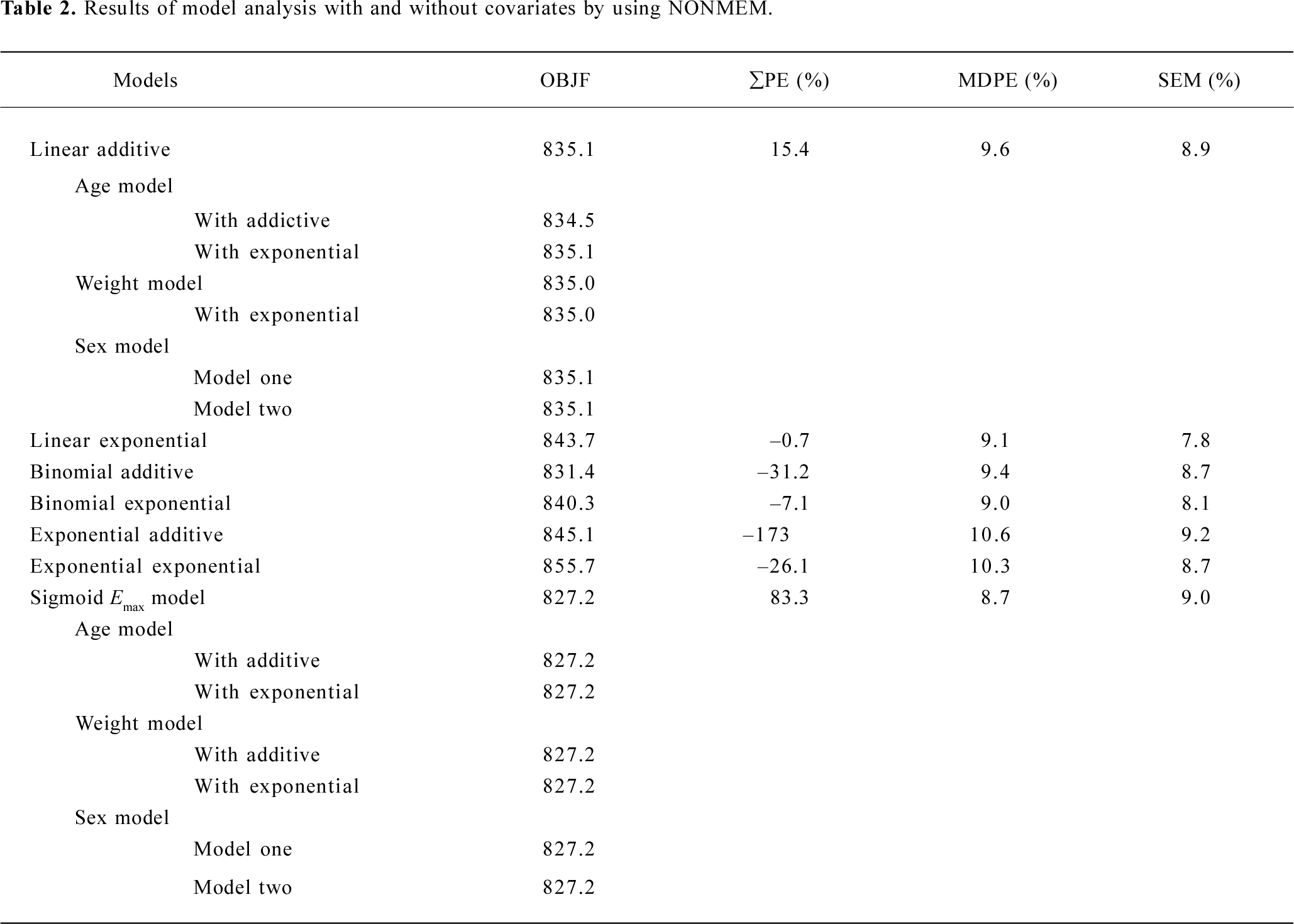
Full table
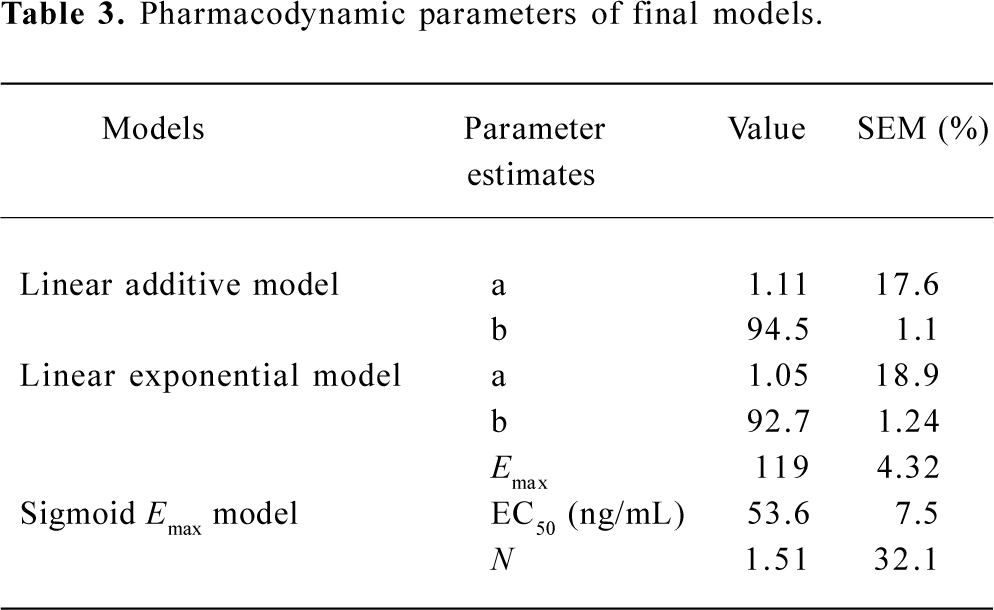
Full table
Model validation To validate the model developed with the data of concentration of propofol in the CSF and the BIS index, we compared the measured CSF propofol concentration with the predicted concentration calculated with the parameters derived from the final model and carried out the linear simulation. To further validate the model we calculated PE (%), MDPE (%) and SEM (%). PE (%) in this case as:
PE= (Cm–Cp)/Cp×100%
where Cm and Cp are the measured and predicted CSF propofol concentration respectively.
Results
Twenty-seven subjects completed the study. Two patients received 6 mg ephedrine boluses during the study because of hypotension, and one received 0.5 mg atropine boluses because of bradycardia. Six patients received positive mask ventilation because of SPO2 values less than 90% (Table 4).
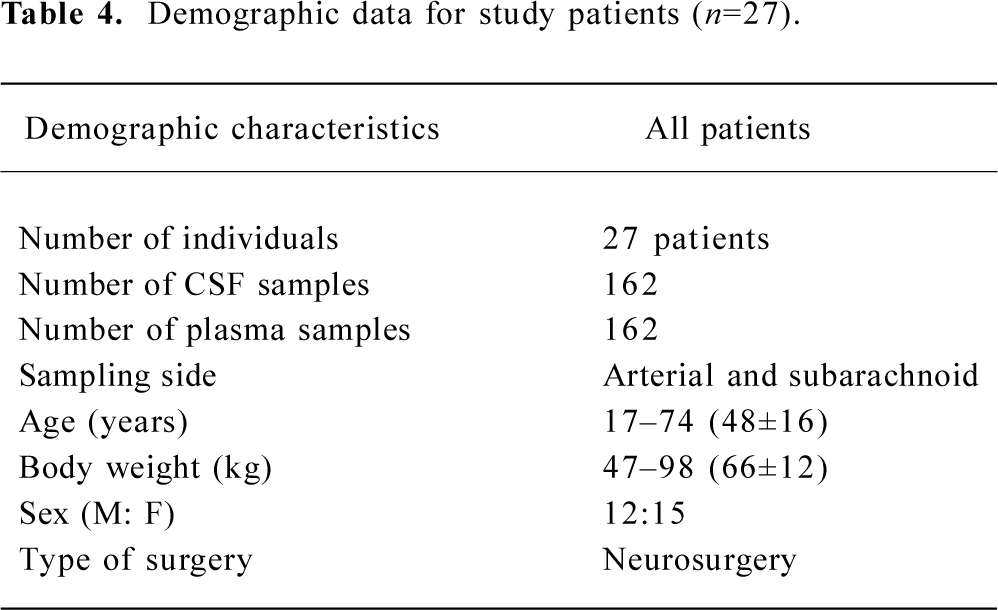
Full table
The lower limit of detection was approximately 5 ng/mL propofol in CSF. The linear range was 5–200 ng/mL and the calibration curve was Y=8789.7x+2332.1, r=0.9994. The coefficient of variation of the high-performance liquid chromatography (HPLC) method did not exceed 10% in the concentration range encountered in the present study.
Pharmacodynamics When OBJF only is considered, we could have also chosen the binomial additive model, but its PE is much greater. After integrated consideration of OBJF, PE, MDPE, and SEM, we selected linear addictive, linear exponential and sigmoid Emax models. As suggested from the OBJF (<3.85, P>0.05), we found no influence of age, weight, and sex on the parameters of models for the analyzed subjects (Table 2).
The estimates of all parameters of final models and their standard errors are summarized in Table 3. The MDPE values of the linear addictive, linear exponential and sigmoid Emax models were 9.6%, 9.1%, and 8.7%, respectively whereas the SEM were 8.9%, 7.8%, and 9.0%, respectively (Table 2).
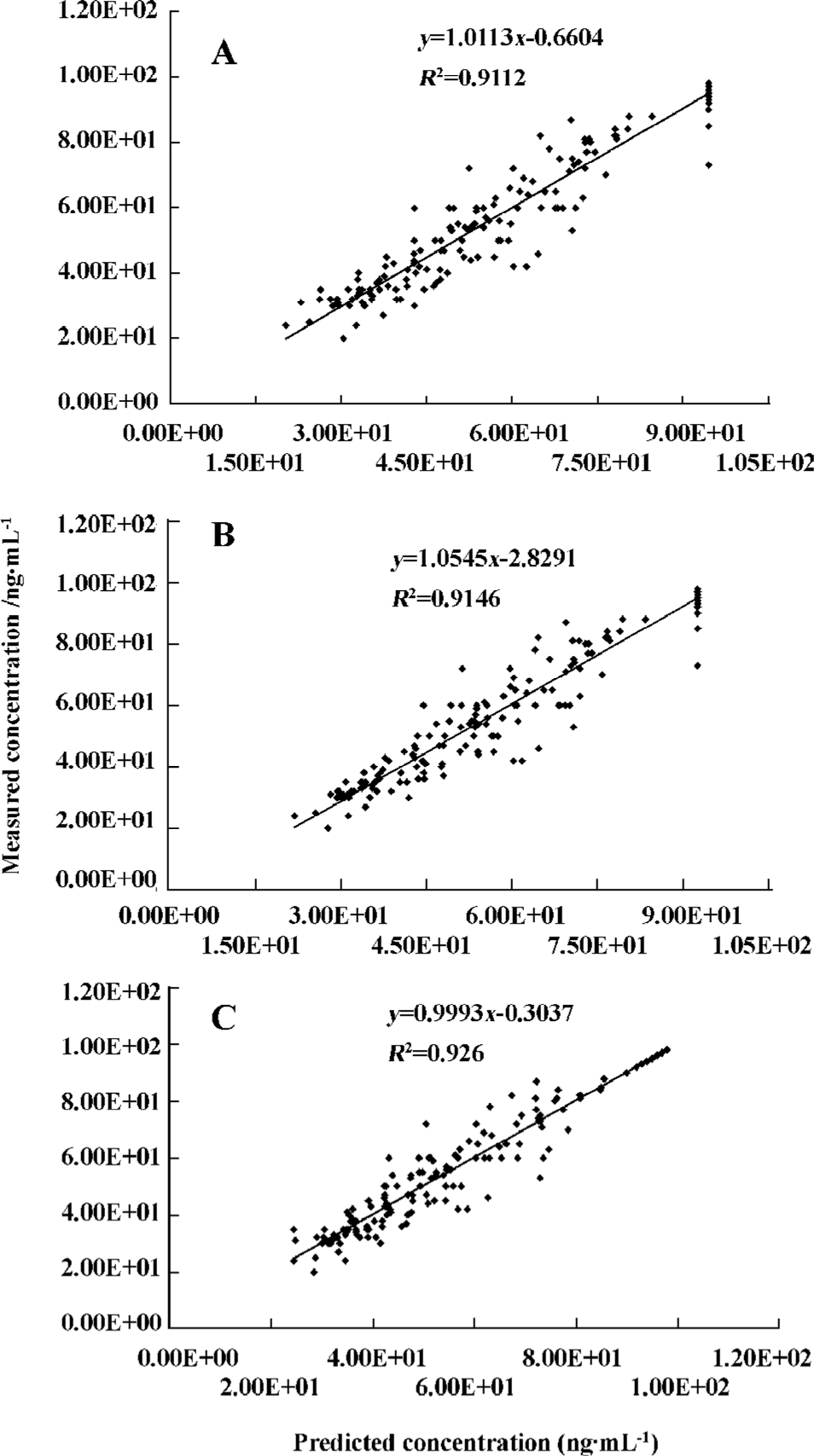
The predicted propofol concentrations in CSF calculated using the parameters derived from the final model were plotted against the measured propofol concentrations (Figure 1A–C). When the linear regression was carried out between the predicted and measured concentrations, the determinant coefficients were 0.911, 0.915, and 0.926 for the linear addictive, linear exponential, and sigmoid Emax models, respectively.
Discussion
In the current study, pharmacodynamic models for CSF concentration versus BIS index in patients scheduled for elective removal of intracranial tumor are presented. BIS index was our electroencephalogram (EEG) measure of drug effect. The concentration of drug in CSF, one of interstitial fluid, can be considered to the effect site concentration of this drug when pharmacodynamic analysis is performed[10]. Previous publications have used effect site concentration rather than plasma concentration to study the pharmacodynamics of propofol[9,11]. CSF may indicate effect site concentrations more accurately than plasma when using target effect-site concentration infusion[12].
One major aim of the present study was to explore whether age, weight, or sex influences the pharmacodynamics of propofol, we therefore incorporated these factors into the model. The results show that these factors have no influence on the pharmacodynamic model determined.
Previous studies have reported conflicting findings concerning the influence of age on the pharmacodynamics of propofol and other anesthetics. In 1985, Homer and Stanski[13] demonstrated that the pharmacokinetics, but not the pharmacodynamics, of thiopental changed with age. In a later study, Stanski and Maitre[14] confirmed these findings. Although the results were controversial, Schnider et al[15] showed that elderly patients were more sensitive to the hypnotic and EEG effects of propofol than were younger persons. An increased sensitivity with age also has been reported for midazolam when the steady-state concentration was used in analysis[2].
Etomidate is also associated with age-related pharmacokinetic changes owing to the increased sensitivity to etomidate in the elderly. However, continuous measurement of EEG median frequency revealed no increased brain sensitivity to etomidate with increasing age[16]. Opioids show the opposite pattern: given that just minor pharmacokinetic differences were found between young and elderly subjects, Scott and Stanski therefore attributed the lower dose requirement for fentanyl and alfentanil in elderly people to increased sensitivity of brain to the drugs[17]. Minto et al[1] found that the equilibration rate constant between plasma and the effect site (keo), and brain sensitivity were all age-dependent, and they concluded that a combination of increased brain sensitivity and decreased clearance accounted for the much smaller remifentanil infusion requirement in the elderly. Lemmens et al[18,19] investigated the effects of age on the pharmacodynamics of alfentanil in 14 younger and 14 older women undergoing curative surgery for primary breast cancer. They found no effect of age on the plasma concentration-effect relation for response to intubation, skin incision, or the probability of needing naloxone at the end of surgery. Ausems et al[20] explored the responses of 37 patients between 23 and 54 years to perioperative stimuli using alfentanil and 66% nitrous oxide in oxygen. They also could not find any relation between pharmacodynamic parameters and the age of subjects.
Conflicting findings have also been reported concerning the influence of sex on the pharmacodynamics of propofol and other anesthetics. Kodaka and colleagues[21] recently examined the effect of sex on the hypnotic requirement for loss of consciousness (LOC) using a volatile (sevoflurane) or an iv (propofol) anesthetic. They found that there was no difference in the BIS at LOC for men and women with either type of hypnotic anesthesia, and concluded that sex had no effect on hypnotic pharmacodynamics. In contrast, Wilhelm et al[22] reported that sex had impact on recovery times and propofol consumption. With propofol-remifentanil anesthesia, if the same amounts of propofol are applied, males awake later; with BIS or Narcotrend monitoring males receive less propofol for comparable EEG effects.
Our finding of no differences in the sensitivity in male and female patients by using the BIS is consistent with the recent work of Kodaka et al[21] for propofol. The BIS index is a combination of 3 parameters derived from analysis of the EEG. The recommended BIS ranges are 65–85 for sedation and 40–45 for general anesthesia[23]. The current version of the BIS index is the result of a search to identify features of the EEG that would reflect the clinical end-points of sedation and hypnosis[23]. It offers several experimental advantages over other clinical measures of drugs, and it is a nearly instantaneous, continuous, objective and reproducible measure of drug effect. Irwin and colleagues[24] showed that the bispectral index correlated well with other clinical end-points and might be useful during propofol anesthesia.
No attempts to model the pharmacokinetic profile were conducted, because the design was not adequate for a kinetic study of a drug such as propofol, which is characterized by fast distribution and 3-compartment model behavior. Because there was no pharmacokinetic model prior to the analysis of the pharmacodynamics, we collected CSF samples from every patient without consideration of time after the administration of propofol.
In conclusion, we used NONMEM to develop 3 kinds of pharmacodynamic models. We found that: (1) propofol concentration in CSF can be used for analysis of pharmacodynamic models; (2) in this small population, age (17–74 years), weight (47–98 kg) and sex do not influence any pharmacodynamic parameters of propofol. However, to verify these preliminary results, a larger study population is required. The results of this study and our companion study about the population pharmacokinetics of propofol[4] suggest that different dose regimens of propofol are probably mainly attributable to pharmacokinetic reasons.
Acknowledgements
Special thanks go to Prof Jian-zhong RUI for his suggestions and guidance during this research.
References
- Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJM, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 1997;86:10-23.
- Jacobs JR, Reves JG, Marty J, White WD, Bai SA, Smith LR. Aging increases pharmacodynamic sensitivity to the hypnotic effects of midazolam. Anesth Analg 1995;80:143-8.
- Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology 2000;92:727-38.
- Li YH, Rui JZ, Zhou YG, Wang LQ, Fu SE, Yang JJ, et al. Population pharmacokinetics of propofol in Chinese patients. Acta Pharmacol Sin 2003;24:581-8.
- Knibbe CA, Zuideveld KP, Aarts LP, Kuks PF, Danhof M. Pharmacokinetics and effects of propofol 6% for short-term sedation in paediatric patients following cardiac surgery. Br J Clin Pharmacol 2002;54:415-22.
- Kearse LA Jr, Rosow C, Zaslavsky A, Connors P, Dershwitz M, Denman W. Bispectral analysis of electroencephalogram predicts conscious processing of information during propofol sedation and hypnosis. Anaesthesiology 1998;88:25-34.
- Malinga M, Peitflaux F, Lepage JY, Malinovsky J, Cozian A, Pinaud M. Dose-response relationship between target-controlled concentration of propofol and bispectral index. Br J Anaesth 1998;80 Suppl:A132.
- Li YH, Bao FP, Zhu SM, Xu JG. Determination of propofol in cerebral spinal fluid by HPLC with fluorescence detection. J Zhejiang Univ 2006;35:87-90. (Medical Science).
- Beal SL, Sheiner LB. NONMEM user's guide. San Francisco: University of California San Francisco; 1998.
- Lin SS, Cai WM, Rui JZ. The new theory and new method for therapeutic drug monitoring. Beijing: Publishing House of Chinese Medicine; 2002.
- Jonsson EN, Karlsson MO. Xpose. An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comp Meth Prog Biomed 1999;58:51-64.
- Luo AL, Yi J, Guo XY, Ren HZ, Huang YG, Ye TH. Concentration of propofol in cerebral spinal fluid: target-controlled infusion. Chin Med J 2004;117:231-4.
- Homer TD, Stanski DR. The effect of increasing age on thiopental disposition and anesthetic requirement. Anesthesiology 1985;62:714-24.
- Stanski DR, Maitre PO. Population pharmacokinetics and pharmacodynamics of thiopental: the effect of age revisited. Anesthesiology 1990;72:412-22.
- Schnider TW, Minto CF, Shafer SL, Andresen C, Goodale DB, Youngs EJ. The influence of age on propofol pharmacodynamics. Aneaesthesiology 1999;90:1502-16.
- Arden JR, Holley FO, Stanski DR. Increased sensitivity to etomidate in the elderly: initial distribution versus altered brain response. Anesthesiology 1986;65:19-27.
- Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther 1987;240:159-66.
- Lemmens HJ, Bovill JG, Hennis PJ, Burm AG. Age has no effect on the pharmacodynamics of alfentanil. Anesth Analg 1988;67:956-60.
- Lemmens HJ, Burm AG, Bovill JG, Hennis PJ. Pharmacodynamics of alfentanil as a supplement to nitrous oxide anaesthesia in the elderly patient. Br J Anaesth 1988;61:173-9.
- Ausems ME, Hug CC, Stanski DR, Burm AGL. Plasma concentrations of alfentanil required to supplement nitrous oxide anesthesia for general surgery. Anesthesiology 1986;65:362-73.
- Kodaka M, Johansen JW, Sebel PS. The influence of gender on loss of consciousness with sevoflurane or propofol. Anesth Analg 2005;101:377-81.
- Wilhelm W, Buchinger H, Biedler A, Altmann S, Larsen R, Kreuer S. Influence of gender on propofol consumption and recovery times. Anaesthesist 2005;54:567-74.
- Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology 2000;93:1336-44.
- Irwin MG, Hui TW, Milne SE, Kenny GN. Propofol effective concentration 50 and its relationship to bispectral index. Anaesthesia 2002;57:242-8.
