Sulfasalazine prevents apoptosis in spermatogenic cells after experimental testicular torsion/detorsion
Introduction
Testicular torsion, which is called spermatic cord torsion, is a serious medical condition that occurs in 1 in 4000 men younger than 25 years of age[1]. If testicular torsion is not treated within 4 to 6 h, spermatogenic cell loss will occur[2,3]. However, even in men who have undergone surgical detorsion within this time period, the ipsilateral testis often becomes permanently dysfunctional[3–5]. Ischemia/reperfusion injury following torsion/detorsion of the testes results in the development of significant pathology[2–4]. Sulfasalazine was first synthesized in 1942 by combining an antibiotic, sulfapyridine, with an anti-inflammatory agent, 5-aminosalicylic acid (5-ASA). Sulfasalazine acts as a potent inhibitor of nuclear factor-kappa B (NF-κB) by inhibiting I kappa B (IκB) phosphorylation, thereby preventing its translocation into the nucleus and decreasing adhesion molecule expression[6–8]. The aim of the present study was to determine if sulfasalazine can prevent the activation of NF-κB in spermatogenic epithelium and prevent the apoptosis of spermatogenic cells after experimental testicular torsion.
Materials and methods
Animals Thirty-two young adult male Sprague-Dawley rats (bodyweight 240–280g) were purchased from the Experimental Animal Center of the Medical Department of Wuhan University. The animals were housed in hanging wire mesh cages, 5 or 6 per cage, under controlled lighting conditions (14-h of light beginning at 6:00 AM, followed by 10-h of darkness) at a temperature of 20–24 °C. They were handled daily for 1 week before the experiment. Rats were divided into 4 groups (A–D), with 8 rats in each group. All drugs and reagents were purchased from Sigma (St Louis, MO, USA). Sulfasalazine, administered at a dosage of 350 mg/kg per d, was dissolved in 15 mL 0.9% sodium chloride solution (SASP solution) for intraperitoneal injection.
Experimental testicular torsion Animals were anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg/kg of bodyweight) for surgery and the duration of ischemia. Testicular torsion was induced as described elsewhere[9]. The testes were retracted through a low midline laparotomy. The left testis of rats in groups A and B were rotated 720° along the longitudinal axis for 2 h. After the torsion was relieved by counter-rotation, the testis was replaced into the scrotum, and blood flow return was observed. The rats of group A(T+SASP) were given intraperitoneal injections of SASP solution just before the incision was closed. After 5 h, when the rats were able to eat food, rats were intraperitoneally injected with the SASP solution every 24 h. The rats of group B(T+SC) were treated as for group A, except that 15 mL 0.9% sodium chloride solution was used instead of SASP. The left testis of rats in groups C and D underwent the same operation as rats in the other two groups, except that testicular torsion was relieved immediately. The rats of group C (SC) received 15 mL 0.9% sodium chloride solution via intraperitoneal injection just before closing cut. After 5 h, when the rats could eat food, we began to intraperitoneally inject the rats with 15 mL 0.9% sodium chloride solution every 24 h. The rats of group D(SASP) were given the SASP solution via intraperitoneal injection just before closing cut. After 5 h, when they all can eat food, we began to intraperitoneally inject the rats with SASP solution every 24 h. At 72 h after repair of torsion, all animals were killed.
Western blotting The torsed/detorsed testes were dissected out and cleaned of adhering fatty and connective tissues, washed with cold 0.9% sodium chloride solution, then cleaned with filter paper. We took out a 3 cm-long piece of seminiferous tubule from the affected testis in order to analyze the change in NF-κB with Western blotting. The rest of each testis was used to measure the apoptosis of spermatogenic cells.
Nuclear proteins were prepared according to a procedure described elsewhere[10]. Seminiferous tubules were treated with ice-cold phosphate-buffered saline (PBS) and scraped. The cell suspension was centrifuged at 1200×g at 4 °C for 8 min. The cell pellets were combined and lysed by resuspension in 100 µL of lysis buffer [10 mmol/L 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), pH 7.9, 60 mmol/L KCl, 1 mmol/L ethylenediaminetetracetic acid (EDTA), 1 mmol/L dithiothreitol (DTT), 1 mmol/L phenyl-methylsulfonylfluoride (PMSF), and 0.5% Nonidet P-40]. A 10 µL aliquot was removed and mixed with an equal volume of trypan blue and examined under a light microscope (×40) to confirm the presence of round, intact nuclei. The remainder of the suspension was centrifuged again. The nuclear pellet was washed with lysis buffer without Nonidet P-40 and centrifuged at 1200×g at 4 °C for 5 min. The pellet was resuspended in 100 µL of nuclear resuspension buffer (25 mmol/L Tris-HCl, pH 8.0, 400 mmol/L KCl, 1 mmol/L DTT, 1 mmol/L PMSF, and 20% w/v glycerol), rapidly frozen and thawed 3 times, and centrifuged at 4000×g at 4 °C for 12 min. The supernatant containing the nuclear proteins was removed, and an aliquot was used for protein determination adapted for microtiter plates.
Ten micrograms of cytoplasmic protein extracts were dissolved in sodium dodecyl sulfate (SDS) buffer, boiled for 5 min, and then subjected to polyacrylamide gel electrophoresis (PAGE) on 10% acrylamide gels. After SDS-PAGE, the gels were transferred to nitrocellulose membranes for 1 h at 4 °C. The blots were blocked with 5% nonfat dry milk in PBS- 0.1% Tween 20 (PBS-T) overnight at 4 °C. Immunological evaluations were then performed for 1 h in PBS-T containing 0.2 µg/mL affinity-purified polyclonal antibodies against the p65 subunits of NF-κB. The blots were subsequently washed with PBS-T and incubated for 1 h with goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (HRP) at a dilution of 1:1000 in PBS-T. After extensive washing with PBS-T, HRP activity was visualized by applying a chemiluminescent substrate then exposing the membrane to an automatic image analysis system. The relative amount (RA) of expression was taken as being proportional to the density of the relevant band on the blot.
Immunohistochemical staining for NF-κB protein Immunohistochemical staining for the NF-κB protein was performed in cells of the seminiferous tubules by using the indirect immunoperoxidase method. The cells were incubated with rabbit polyclonal antibody against the p65 subunit of NF-κB at a dilution of 1:200 in PBS for 1 h at room temperature and then incubated for 1 h with biotinylated goat anti-rabbit IgG at a dilution of 1:500. The bound antibody was visualized with avidin-biotin complexes. The slides were counterstained with hematoxylin. After being mounted, every slide was viewed with an Olympus microscope. For evaluation of NF-κB activation, microscopic fields (×200) were selected at random. The cells that were activated were stained yellow. The percentage of NF-κB-positive nuclei was determined by counting 100 cells from the spermatogenic epithelium.
Analysis of apoptosis of spermatogenic cells The torsed testes were washed in cold 0.9% sodium chloride solution, and then cleaned with filter paper. Tissue sections were stored in formaldehyde solution for 1 d, then embedded in paraffin. Five-micrometer-thick sections were mounted on silan-coated glass slides and fixed for one day at 65 °C.
Endogenous peroxidase was inactivated by 0.3% H2O2 for 30 min at room temperature. To make the sections permeable, they were incubated with a permeabilization buffer for 5 min at 4 °C. In situ end-labeling was performed by using an In Situ Apoptosis Detection Kit (Boehringer Mannheim Corp, Mannheim, Germany) composed of nonradioactive fluorescein-dideoxyuridine triphosphate. The sections were incubated with terminal deoxynucleotidyl transferase and fluorescein-dideoxyuridine triphosphate at 37 °C for 90 min in a humidified chamber, and the 3'-OH ends of the DNA fragments were tailed with fluorescein. The sections were then washed 3 times in PBS. After being incubated with anti-fluorescein isothiocyanate horseradish peroxidase conjugate for 30 min at 37 °C, the slides were washed 3 times in PBS, developed with 0.05% diaminobenzidine, and stained for 15 min at room temperature. The specimens were then washed 3 times in distilled water, counterstained with Mayer hematoxylin solution for 10 min, dehydrated, mounted, and viewed with an Olympus microscope.
For evaluation of apoptosis, microscopic fields (×200) were selected at random. Apoptotic spermatogenic cells were stained yellow. The percentage of apoptotic spermatogenic cells, the apoptosis index (AI), was determined by counting 100 cells from the spermatogenic epithelium.
Statistical analysis Data are presented as mean±SD. Autoradiographic analyses were repeated 3 times. The significance of the differences between means was determined by using Student’s t-test, ANOVA and the Dunnett t-test. Statistical significance was set at P<0.05, and the marked statistical significance was set at P<0.01.
Results
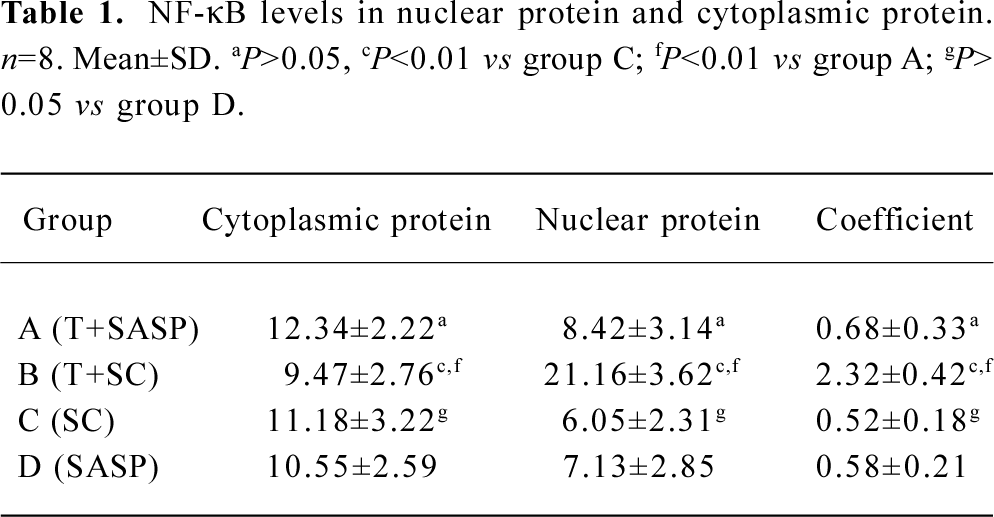
Investigation of NF-κB protein levels To elucidate the mechanisms responsible for altered susceptibility of spermatogenic cells to apoptosis, we investigated the NF-κB protein levels in nuclear protein and cytoplasmic protein. There was no significant difference (P>0.05) in NF-κB protein levels not only in nuclear protein (t=1.405), but also in cytoplasmic protein (t=1.294) between the spermatogenic epithelium of groups C and D; and likewise, there was no significant difference (P>0.05) in NF-κB protein levels not only in nuclear protein (t=1.615) but also in cytoplasmic protein (t=1.144) between the spermatogenic epithelium of groups A and C. However, a markedly significant increase (F=9.56, P<0.01) in NF-κB protein levels was noted in nuclear protein in the spermatogenic epithelium of group B compared with groups A and C, but no significant difference (F=1.92, P>0.05) in NF-κB protein levels was noted in cytoplasmic protein in the spermatogenic epithelium of group C compared with group D (Figure 1, Table 1).
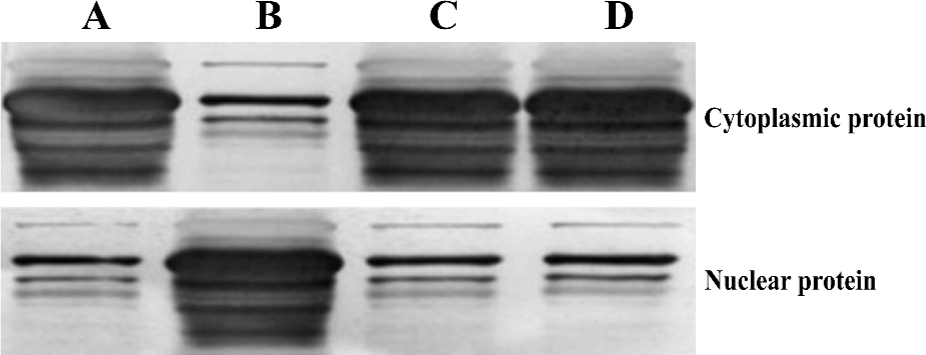
Full table
We obtained NF-κB coefficients (C) according to the method previously described by Thiele et al[11], using the equation C=NU/CY, where NU is the NF-κB protein level in nuclear protein and CY is the NF-κB protein level in cytoplasmic protein. There was no significant difference (t=0.995, P>0.05) in the NF-κB coefficient for the spermatogenic epithelium of groups C and D, and no significant difference (t=1.182, P>0.05) in the NF-κB coefficient for spermatogenic epithelium of groups A and C. There was, however, a markedly significant increase (F=10.27, P<0.01) in the NF-κB coefficient for nuclear protein in the spermatogenic epithelium of group B compared with groups A and C (Table 1).
Immunohistochemical staining for NF-κB protein To elucidate the role of NF-κB protein in the apoptotic process, we investigated its level of expression and cellular distribu-tion. We found that almost all NF-κB protein was in the cytoplasm in groups A, C and D. The NF-κB protein levels in the nuclear protein were much greater than those in the cytoplasmic protein in group B (Figures 2 and 3). There was no significant difference (t=0.834, P>0.05) in the proportion of cells with NF-κB-positive nuclei in the spermatogenic epithelium of groups C and D, and there was no significant difference (t=1.711, P>0.05) in the proportion of cells with NF-κB-positive nuclei in the spermatogenic epithelium of groups A and C. There was, however, a markedly significant increase (F=7.19, P<0.01) in the proportion of cells with NF-κB-positive nuclei in the spermatogenic epithelium of group B compared with groups A and C (Table 2). We found that cells with NF-κB-positive nuclei were always spermatogonia or spermatocytes, and few Sertoli cells, Leydig cells, or endothelial cells changed.
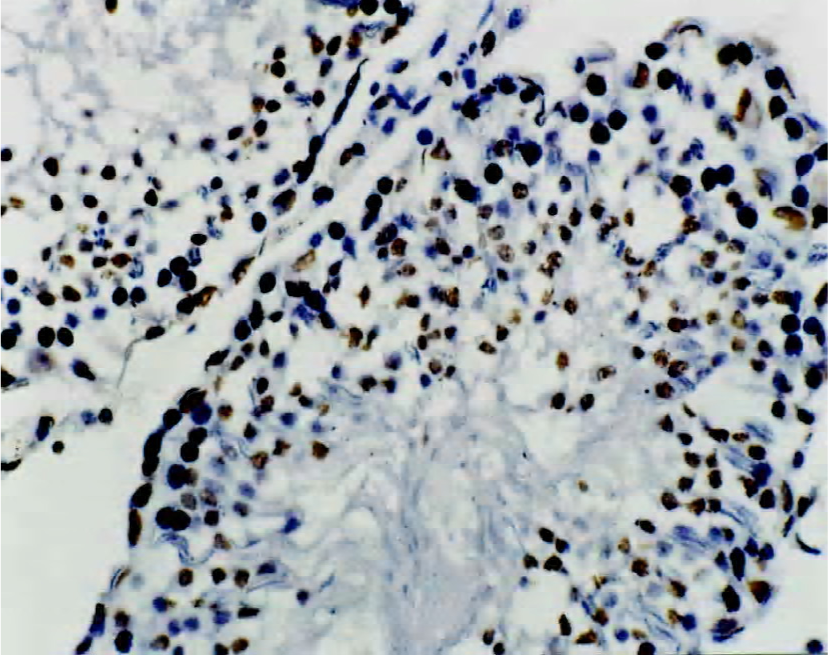
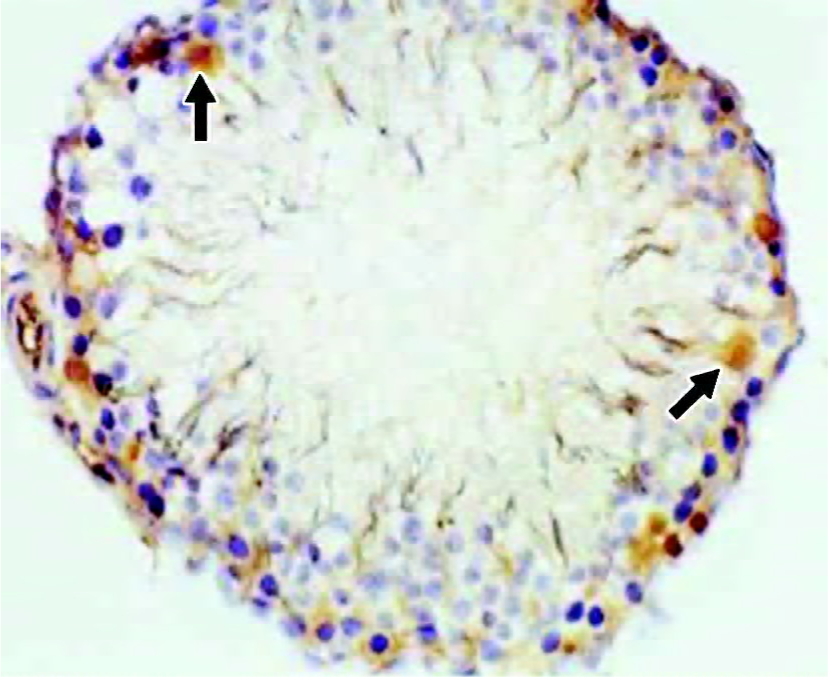
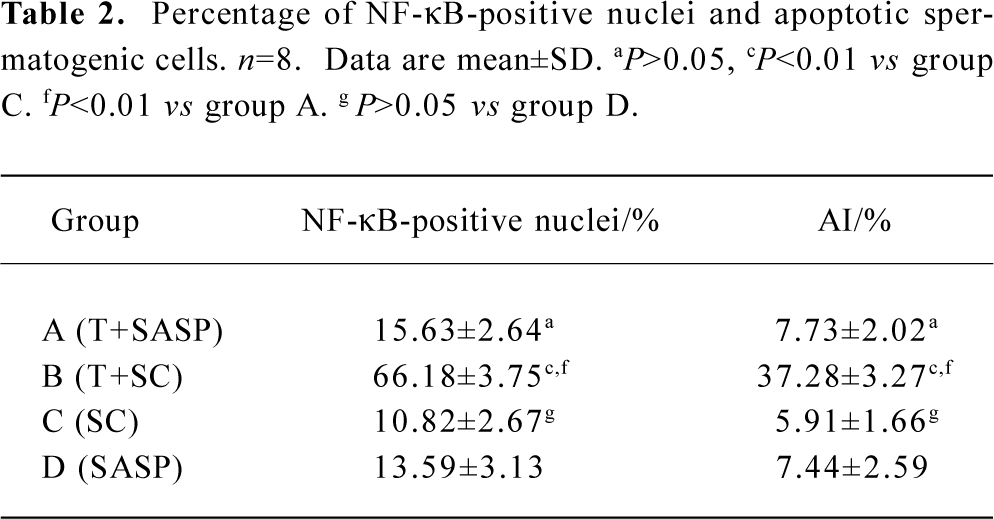
Full table
Apoptosis of spermatogenic cells In order to evaluate the apoptosis of spermatogenic cells in the spermatogenic epithelium, we analyzed the AI of spermatogenic cells by using the TUNEL technique. There was no significant difference (t=0.962, P>0.05) in AI in the spermatogenic cells of groups C and D, and there was no significant difference (t=1.699, P>0.05) in AI in the spermatogenic cells of groups A and C. There was a markedly significant increase (F=8.41, P<0.01) in AI in the spermatogenic cells of group B compared with group A and C (Figures 4 and 5, Table 2). Apoptotic spermatogenic cells were always spermatogonia or spermatocytes, and not Sertoli cells, Leydig cells or endothelial cells.
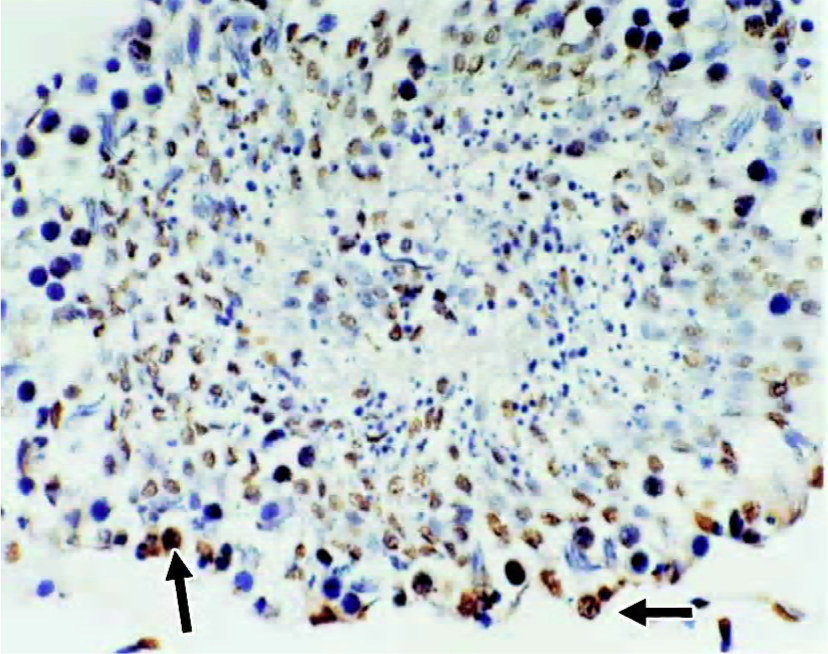
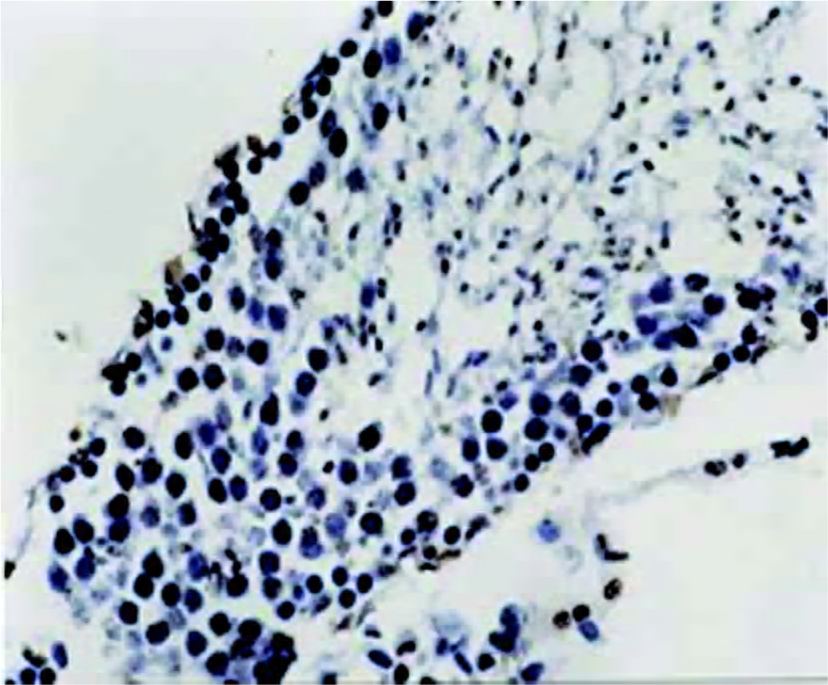
Discussion
Testicular torsion results from an inadequate fixation of the testicular mesorchium, leading to hypermobility of the testis[1,3,4,12]. The degree of tissue injury is directly proportional to the degree of testicular ischemia[3,4,13]. Experimental testicular torsion induces testicular ischemia during torsion. Relief of torsion allows reperfusion of the tissue. Testicular torsion causes ischemia and reperfusion injury that results in spermatogenic cell loss. Loss of spermatogenic cells after reperfusion is caused by spermatogenic-cell-specific apoptosis. The spermatogenic-cell-specific apoptosis ultimately results in male infertility[3,14–16].
According to Weins and Lucchesi[17], reperfusion injury is induced by ROS, which arise either from activation of the xanthine oxidase system in parenchymal cells or from leukocytes that first adhere to the reperfusing venule wall before undergoing diapedesis into the tissue itself. Torsed testes torsion undergo ischemia and reperfusion injury that results in increased adhesion molecule expression, leukocyte migration, and damage to the spermatogenic epithelium[14–16]. Following reperfusion, ROS are released from ischemic tissues and produce local damage, resulting in an upregulation of endothelial cell surface proteins. The increase in endothelial proteins and local vasodilation promotes the binding of neutrophils and infiltration of the damaged tissue[16,17]. The neutrophils promote necrosis and additional injury with the release of more ROS, thereby propagating an ongoing cycle of reperfusion injury[2,4,14,17].
Previous studies have demonstrated that NF-κB played a vital role in ischemia-reperfusion injury events[18–21]. NF-κB is a transcription factor associated with immunomodulation of the immune response. In its quiescent state, NF-κB is a heterodimer consisting of the p50 and p65 (Rel A) subunits that remain in the cytoplasm bound to a family of inhibitory proteins that are collectively termed IκB. NF-κB is activated through phosphorylation and subsequent degradation of IκB. Many agents, including ROS, interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α) are sufficient to cause IκB phosphorylation[22–25]. Once activated, the nuclear localization signal of NF-κB is exposed and NF-κB is translocated into the nucleus. NF-κB transcriptionally activates many genes involved in the inflammatory process, including intercellular adhesion molecule 1 (ICAM-1), vascular cellular adhesion molecule 1 (VCAM-1), and E-selectin (ELAM-1), which are important mediators of the inflammatory process in reperfusion injury[25–29]. Apoptosis always occurs in the ischemic-reperfused tissue, and the functions of the tissue never remain unchanged[14–16]. Our study was designed to investigate the importance of NF-κB in testis ischemia-reperfusion injury. We hypothesized that the increase in free radical production (in particular ROS) during testicular torsion activated NF-κB as well as increased the host response, leading to increase in the apoptosis of spermatogenic cells.
Our data suggest the importance of NF-κB in testis ischemia-reperfusion injury, and further suggest the novel use of sulfasalazine as an inhibitor of NF-κB following testes torsion. Sulfasalazine is currently used to treat inflammatory bowel disease and rheumatoid arthritis[6,7]. However, despite being in common use for the past 50 years, its mechanism of action remains undefined. Recently, sulfasalazine has been found to have numerous biological effects, including immunosuppressive and modulatory actions on lymphocytes and leukocyte function. Sulfasalazine inhibits IL-2 synthesis and IL-1 production in lymphocytes[6,8,29]. Other studies have demonstrated that salicylates inhibited IκB phosphorylation to block inflammatory effects[6,8]. Wahl et al[6] found that sulfasalazine acted as a potent inhibitor of NF-κB by inhibiting IκB phosphorylation, thereby preventing its translocation into the nucleus and decreasing adhesion molecule expression. In addition, sulfasalazine has been shown to act as a free radical scavenger[7,29]. We compared the NF-κB coefficient and AI of the 4 groups, and found that there was no significant difference in the NF-κB coefficient or AI in the spermatogenic epithelium between groups C and D, or between groups A and C, but a markedly significant increase in both was noted in the spermatogenic epithelium of group B compared with groups A and C. This finding shows that administration of sulfasalazine when no testicular torsion has occurred does not cause increases in the NF-κB coefficient or AI; however, when testicular torsion has occurred, administration of sulfasalazine can prevent increases in the NF-κB coefficient and AI. We also investigated the NF-κB protein levels in the nuclear protein and cytoplasmic protein of the spermatogenic epithelium of the 4 groups, and found that when no testicular torsion occurred, NF-κB protein was not translocated from the cytoplasm into the nucleus, and administration of sulfasalazine did not cause this translocation. However, when testicular torsion occurred, NF-κB protein was activated; after torsion, NF-κB protein levels increased, particularly in the nuclear protein, then led to increases in the apoptosis of spermatogenic cells. Administration of sulfasalazine can prevent the activation of NF-κB protein, and then also prevent increases in AI. We also found that cells with NF-κB-positive nuclei were always spermatogonia or spermatocytes, which were also always the apoptotic cells. Conversely, the cells that were seldom activated, for example Sertoli cells, Leydig cells, and endothelial cells, were seldom apoptotic. This finding demonstrates that NF-κB activation is important in the apoptosis process. Treatment with sulfasalazine prior to detorsion decreases the AI close to the levels of the negative controls, suggesting that sulfasalazine can protect against results that stimulate the NF-κB pathway and decrease the concentration of ROS present in testicular torsion, which would also decrease adhesion molecule expression. In conclusion, we found that NF-κB played a vital role in the ischemia-reperfusion immunology of testicular torsion and we discovered a novel use for the anti-inflammatory agent sulfasalazine. Treatment with sulfasalazine was able to decrease reperfusion injury by preventing increases in AI in the testicular torsion model. Sulfasalazine inhibits the activation of NF-κB, and is an inexpensive and safe agent that could have an important role in the ischemia-reperfusion drug regimen to decrease episodes of apoptosis. Sulfasalazine may be beneficial to decrease morbidity and infertility following testis torsion.
References
- Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol 1989;142:746-8.
- Consentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histopathology of prepubertal rat testes subjected to various duration of spermatic cord torsion. J Androl 1986;7:23-31.
- Anderson JB, Williamson RCN. The fate of the human testes following unilateral torsion of the spermatic cord. Br J Urol 1986;58:698-704.
- Turner TT, Brown KJ. Spermatic cord torsion. Loss of spermatogenesis despite return of blood flow. Biol Reprod 1993;49:401-9.
- Williamson RCN. The continuing conundrum of testicular torsion. Br J Surg 1985;72:509-10.
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest 1998;101:1163-74.
- Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 2002;33:311-22.
- Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit IκB-α phosphorylation, endothelial-leukocyte adhesion molecule expression, neutrophil transmigration. J Immunol 1996;156:3961-9.
- Prillaman HM, Turner TT. Rescue of testicular function after acute experimental torsion. J Urol 1997;157:340-5.
- Ye ZS, Samuels HH. Cell-and sequence-specific binding of nuclear protein to 5’-flanking DNA of the rat growth hormone gene. J Biol Chem 1987;262:6313-7.
- Thiele K, Bierhaus A, Autschbach F, Hofmann M, Stremmel W, Thiele H, et al. Cell specific effects of glucocorticoid treatment on the NF-κB/IκBα system in patients with Crohn’s disease. Gut 1999;45:693-704.
- Gupta G, Srivastava A, Setty BS. Activities and androgenic regulation of Kreb cycle enzymes in the epididymis and vas deferens of rhesus monkey. Endocr Res 1994;20:275-90.
- Smith RD. Testicular torsion: time is the enemy. ANZ J Surg 2002;72:316.
- Turner TT, Tung KSK, Tomosnasa H. Acute testicular ischemia results in germ cell specific apoptosis in the rat. Biol Reprod 1997;57:1267-74.
- Liu X, Zheng X, Li S, Hu L, Zheng H. Testicular torsion/detorsion induced germ cell apoptosis and expression of gene iNOS. Chin J Androl 2001;15:11-3. Chinese..
- Liu Z, Zheng X, Li S, Yang Z, Hu L. Effect of antioxidant enzyme and lipid peroxidation on germ cell apoptosis in testicular torsion/detorsion of rats. Med J Wuhan Univ 2003;24:161-4. Chinese..
- Weins SW, Lucchesi BR. Free radicals and ischemic tissue injury. Trends Physiol Sci 1990;11:11848-52.
- Kacimi R, Karliner JS, Koudssi F, Long CS. Expression and regulation of adhesion molecules in cardiac cells by cytokines. Response to acute hypoxia. Circ Res 1998;82:576-86.
- Cooper M, Lindholm P, Pieper G, Seibel R, Moore G, Nakanishi A, et al. Myocardial nuclear factor-kappa B activity and nitric oxide production in rejection cardiac allografts. Transplantation 1998;66:838-44.
- Siebenlist U, Franzoso G, Brown K. Structure, regulation, and function of NF-κB. Annu Rev Cell Biol 1994;10:405-55.
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell 1996;87:13-20.
- Beg AA, Baldwin AS Jr. The IκB proteins: multifunctional regulator of Rel/NF-κB transcription factors. Genes Dev 1993;7:2064-70.
- Stancovski I, Baltimore D. NF-kappaB activation: the IkappaB kinase revealed? Cell 1997;91:299-302.
- Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappaB and IkappaB alpha: an inducible regulatory system in endothelial activation. J Exp Med 1994;179:503-12.
- Baldwin AS Jr. The NF-κB and IκB proteins: new discoveries and insights. Ann Rev Immunol 1996;14:649-83.
- Tosa Y, Kollias N, Lee WP, May JW Jr. Reduction of ischemia-reperfusion injury by monoclonal antibody to intercellular adhesion molecule-1. Transplant Proc 1996;28:1210-1.
- Blackwell TS, Christman JW. The role of nuclear factor-κB in cytokine gene regulation. Am J Respir Cell Mol Biol 1997;17:3-9.
- Zhu Z, Tang W, Gwaltney JM Jr, Wu Y, Elias JA. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol 1997;273:814-24.
- Feeley BT, Park AK, Hoyt EG, Robbins RC. Sulfasalazine inhibits reperfusion injury and prolongs allograft survival in rat cardiac transplants. J Heart Lung Transplant 1999;18:1088-95.
