Influence of low dietary histamine on the seizure development of chemical kindling induced by pentylenetetrazol in rats1
Introduction
Since Churchill first described certain antihistaminics enhance epileptic extent[1], a number of studies have indicated that brain histamine is involved in mechanisms regulating seizure susceptibility, and the anticonvulsant action of histamine has been well documented[2–5]. Histamine increases the threshold for amygdaloid kindling and pentylenetetrazol (PTZ)-induced seizures[6,7]. A marked increase in H1-receptor binding has been observed around the epileptic foci of complex partial seizures and may be involved in inhibiting the spread of seizure activity[8]. In addition, we have recently reported severe seizure development of PTZ-induced kindling in H1 receptor knockout mice (H1R KO) and histidine decarboxylase-deficient (HDC -/-) mice[9].
It has recently been reported that long-term treatment with high histamine level diets could increase brain histamine levels in HDC -/- mice, which lack the ability to synthesize histamine[11,12]. These results suggest that histamine can be absorbed from the digestive tract and distributed to the brain[12]; furthermore, histamine levels in various diets might influence the histaminergic system and so affect certain neuronal functions, such as epilepsy. Yet, so far there is only limited information about the effects of dietary histamine on these neurotransmitters.
Kindling has been accepted as an experimental animal model for analyzing epilepsy and epileptogenesis, and estimating the effectiveness of antiepileptic drugs. It is generally known that PTZ-induced kindling creates proconflict and convulsant effects in rodents, and is considered as an adequate model of human absence epilepsy and myoclonic, generalized tonic-clonic seizure[13]. We therefore designed the present study to further elucidate the possible relationship between the appearance of seizures and histamine in daily diet.
Materials and methods
Animals Male Sprague-Dawley rats (4–5 weeks old) were purchased from Japan SLC (Shizuoka, Japan), and were maintained in individual cages under constant temperature (22–24 °C) and humidity (40%–70%) with a 12-h light/dark cycle (lights on from 6:00 AM–18:00 PM). The rats were fed with basal histamine diets (BH, containing 79.6 nmol/g of histamine, Nihon Nosan Kogyo KK, Yokohama, Japan) or low histamine diets (LH, containing 1.45 nmol/g of histamine, Ver 3 from Nihon Nosan Kogyo KK, Yokohama, Japan), and water was given ad libitum. Behavioral studies were carried out each day between 10.00 AM and 17.00 PM. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemical kindling After 2 weeks of LH feeding, the rodents were intraperitoneally injected with PTZ (35 mg/kg) every 48 h until the animals were fully kindled[4,5,14]. After PTZ injections, the seizure intensity was scored for 30 min using the following scale: Stage 0, no response; Stage 1, ear and facial twitching; Stage 2, myoclonic body jerks; Stage 3, clonic forelimb convulsions; Stage 4, generalized clonic convulsions, turn over on to side position; Stage 5, generalized clonic-tonic convulsions or died within 30 min. In addition, the latency to the onset of myoclonic jerks were measured and analyzed statistically. In the absence of seizures within 30 min, the latency was taken as 1800 s. When the rat had a seizure score of 4 or 5 at three consecutive injections, it was defined as fully kindled.
Measurements of brain histamine The rodents were killed by decapitation. The brain was quickly removed, and placed on an ice-cold stainless steel plate, and dissected according to the methods of Glowinski and Iversen[15]. These tissues were stored at -80 ºC until assayed. The brain tissue was weighed and homogenized in 3% perchloric acid. The homogenate was centrifuged at 15 000×g for 20 min at 4 ºC to obtain a clear supernatant. After filtration (0.22 μm), histamine was analyzed fluorometrically with o-phthaladehyde after separation on an HPLC system (CCP & 8010 series, Tosoh, Tokyo, Japan, particle size 5 μm). The fluorescence intensity was measured at 450 nm with excitation at 360 nm in a spectrofluorometer (model C-R3A, Shimadzu, Kyoto, Japan)[9,10].
Statistical analysis All data were expressed as mean± SEM. One-way analysis of variance with Dunnetts test was used for calculating a significant difference. Statistical significance was set at P<0.05.
Results
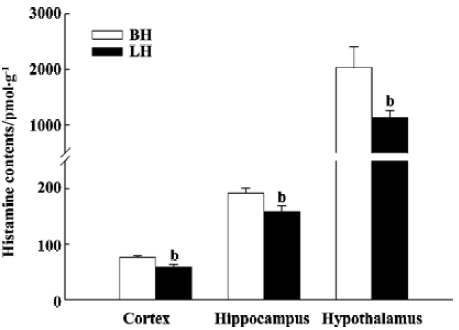
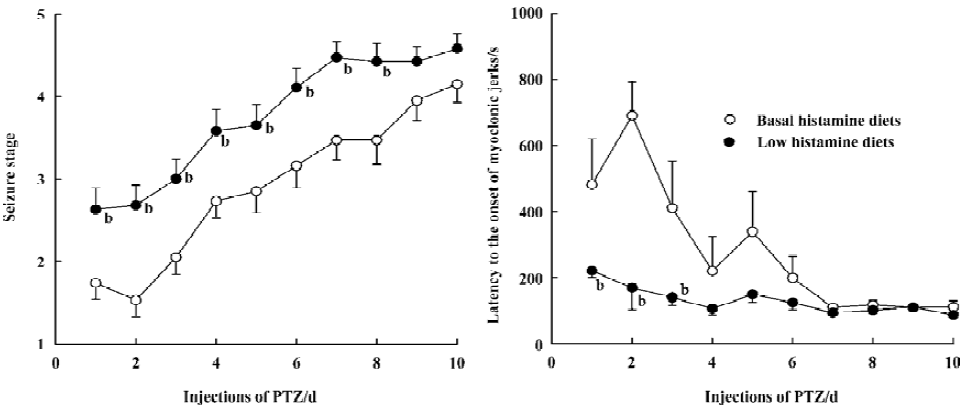
Effects of LH diet on seizure development induced by PTZ-kindling After 2 weeks on a LH diet, compared with BH, histamine levels significantly decreased in the cortex (23.2%), hippocampus (16.5%), and hypothalamus (43.7%) (P<0.05) (Figure 1). Rats fed with the LH diet for 2 weeks showed greatly enhanced development of PTZ-induced kindled seizures (Figure 2). They were fully kindled through less chemical stimuli compared with those fed with the BH diet. Rats fed with the LH diet and control rats fed with the BH diet became fully kindled at d 8 and d 14, respectively. In addition, rats fed with the LH exhibited more severe seizure stages than rats fed with the BH diet (P<0.05 for the first 8 d of PTZ injection). The latency to the onset of seizures was also significantly shorter in the LH group (P<0.05 for the first 3 d of PTZ injection).
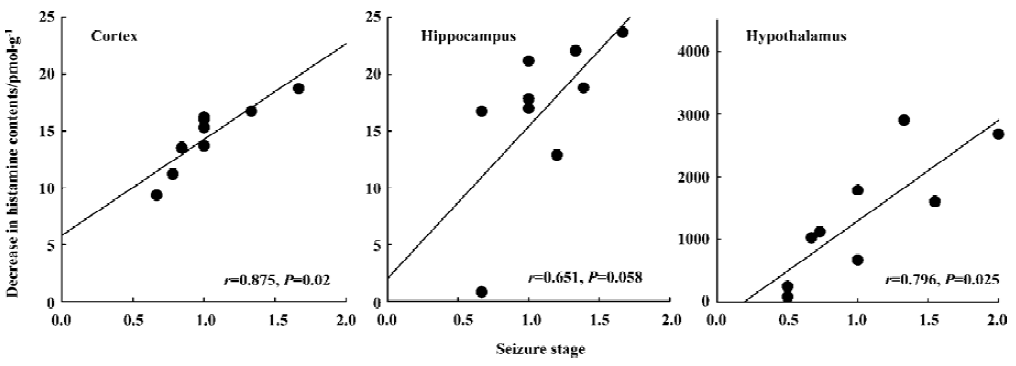
Correlation between the decrease in brain histamine levels and the increase in seizure stage after the first PTZ injection To investigate the relationship between the increase in seizure scores and the decrease in the histamine levels in the brain induced by the LH diet, the regression line of Y (decrease in histamine levels of the cortex, hippocampus, and hypothalamus) on X (increase in seizure scores) and the correlation coefficient (r) were calculated (Figure 3). The correlation coefficients of the cortex, hippocampus, and hypothalamus were 0.875, 0.651, and 0.796, respectively.
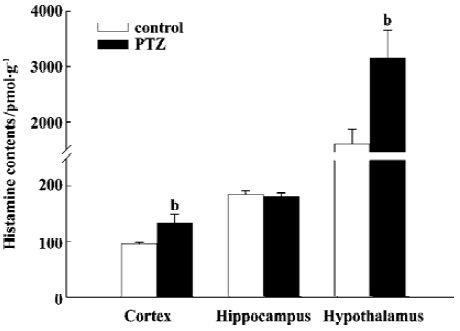
Effects of chronic kindled seizures on levels of histamine in the brain of rats Twenty-four hours after fully kindled, the rats were killed by decapitation. The histamine in the brain tissues was measured. Compared with control rats fed the LH diet without PTZ treatment, chronic PTZ kindled elicited significant increases in histamine levels both in the cortex (137.6%) and hypothalamus (195.7%), but not in the hippocampus (Figure 4).
Discussion
The interactions between histamine levels in food and normal physiological functions have recently excited interest[11,12]. This study provides the first evidence that dietary histamine affects the onset and development of seizures induced by chronic PTZ kindling in rats. Relative to BH diets, LH diets significantly augmented the onset of seizures induced by chronic PTZ-kindling, and further resulted in facilitation of subsequent PTZ kindling process. Meanwhile, significant decreases in histamine levels in the cortex, hippocampus, and hypothalamus were observed following the 2-week LH diets. Interestingly, a strong correlation was found between decreased histamine levels in the cortex and hypothalamus and increased seizure scores following PTZ treatment. It is therefore likely that the reduced histamine plays an important role in the seizure susceptibility. We previously reported that α-fluoromethylhistidine, an inhibitor of HDC, accelerates seizure development induced by PTZ- kindling in rats[4].
The chronic kindled seizures markedly increased histamine levels in the cortex and hypothalamus in rats fed with the LH diet. It has been reported that increased histamine levels in the cortex and hypothalamus are induced by PTZ kindling seizures in H1R KO mice[9], and by maximal electroshock in mice[16]. However, the histamine levels significantly decrease in the amygdala of electrical stimulation site after the development of amygdaloid kindling[6,17,18] and in the cortex and hypothalamus following acute PTZ kindled seizure[16]. These differences might be due to variations in the experimental methods, the species used, drug dosages, or routes of administration. PTZ kindling is the animal model for human primary generalized (or absence) epilepsy. Our data at least suggest that the chronic development of this epileptic seizure is different from the acute one[16], and other epilepsy styles, such as temporal lobe epilepsy, generalized seizure, and so on[16,17]. At present, we have no explanation for why chronic kindling could increase histamine levels in rats fed with LH diets. Further experiments are needed to elucidate its mechanism. An increase in histamine levels in the cortex and hypothalamus might reflect a compensating and protective physiological mechanism after long-term low levels of histamine induced by LH diets. These results further support the concept of histamine as an endogenous anticonvulsant.
An interesting finding in the present study is that there was no significant changes of histamine levels in the hippocampus in the fully PTZ-kindled rats. This suggests that once it is fully kindled by PTZ, the type of seizure is less sensitive to histamine levels in the hippocampus, although it is well known that the hippocampus plays an important role in electrically kindled seizures[17,19,20]. Furthermore, we have also reported that PTZ-kindled is independent of the hippocampal N-methyl-D-aspartate receptor subunit 2B, which was strongly associated with amygdaloid kindled seizures in rats[9]. These results suggest that the different types of kindled seizures may be associated with region-dependent foci and circuits, and therefore results from different mechanisms. For example, Mirski et al found that separate neuronal circuits mediate PTZ and maximal electroshock seizures[21].
Therefore, the present study indicates that histamine in daily food can influence histaminergic function in the brain, and may play an important role in regulating human primary generalized (or absence) epilepsy. These findings further suggest that more attention should be paid to daily dietary containing low and high histamine, and their possible influence on seizure susceptibility of human primary generalized (or absence) epilepsy.
Acknowledgement
The authors thank Dr Iain C BRUCE for critically reading this paper.
References
- Churchill JA, Gammon JD. The effect of antihistaminic drugs on convulsive seizures. J Am Med Assoc 1949;141:18-21.
- Lintunen M, Sallmen T, Karlstedt K, Fukui H, Eriksson KS, Panula P. Postnatal expression of H1-receptor mRNA in the rat brain: correlation to L-histidine decarboxylase expression and local upregulation in limbic seizures. Eur J Neurosci 1998;10:2287-301.
- Toyota H, Ito C, Yanai K, Sato M, Watanabe T. Histamine H1 receptor binding capacities in the amygdala of the amygdaloid kindled rat. J Neurochem 1999;72:2177-80.
- Zhang LS, Chen Z, Huang YW, Hu WW, Wei EQ, Yanai K. Effects of endogenous histamine on seizure development of pentylenetetrazole-induced kindling in rats. Pharmacology 2003;69:27-32.
- Zhang LS, Chen Z, Ren KM, Leurs R, Chen JC, Zhang WB, et al. Effects of clobenpropit on pentylenetetrazole- kindled seizures in rats. Eur J Pharmacol 2003;482:169-75.
- Kamei C, Ishizawa K, Kakinoki H, Fukunaga M. Histaminergic mechanisms in amygdaloid-kindled seizure in rats. Epilepsy Res 1998;30:187-94.
- Scherkl R, Hashem A, Frey HH. Histamine formation in rat brain: its role in regulation of seizure susceptibility. Epilepsy Res 1991;10:111-8.
- Iinuma K, Yokoyama H, Otsuki T, Yanai K, Watanabe T, Ido T, et al. Histamine H1 receptors in complex partial seizure. Lancet 1993;341:238.
- Chen Z, Li ZY, Sakurai E, Mobarakeh JI, Ohtsu H, Watanabe T, et al. Chemical kindling induced by pentylenetetrazol in histamine H1 receptor gene knockout mice (H1KO), histidine decarboxylase-deficient mice (HDC-/-) and mast cell-deficient W/Wv mice. Brain Res 2003;968:162-6.
- Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr 1985;344:115-23.
- Ohtsu H, Kuramasu A, Tanaka S, Terui T, Hirasawa N, Hara M, et al. Plasma extravasation induced by dietary supplemented histamine in histamine-free mice. Eur J Immunol 2002;32:1698-708.
- Watanabe T, Yanai K. Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. Tohoku J Exp Med 2001;195:197-217.
- Morimoto K, Fahnestock M, Racine R J. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol 2004;73:1-60.
- Chen Z, Li WD, Zhu LJ, Shen YJ, Wei EQ. Effects of histidine, a precursor of histamine, on pentylenetetrazole-induced seizures in rats. Acta Pharmacol Sin 2002;23:361-6.
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 1966;13:655-69.
- Vohora D, Pal SN, Pillai KK. Histamine and selective H3-receptor ligands: a possible role in the mechanism and management of epilepsy. Pharmacol Biochem Behav 2001;68:735-41.
- Toyota H, Ito C, Ohsawa M, Sakurai E, Sato M, Watanabe T. Decreased central histamine in the amygdaloid kindling rats. Brain Res 1998;802:241-6.
- Chen Z, Sakurai E, Hu WW, Jin CL, Kiso Y, Kato M, et al. Pharmacological effects of carcinine on histaminergic neurons in the brain. Br J Pharmacol 2004;143:573-80.
- Simonato M, Hosford DA, Labiner DM, Shin C, Mansbach HH, McNamara JO. Differential expression of immediate early genes in the hippocampus in the kindling model of epilepsy. Brain Res Mol Brain Res 1991;11:115-24.
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 1999;89:157-66.
- Mirski MA, McKeon AC, Ferrendelli JA. Anterior thalamus and substantia nigra: two distinct structures mediating experimental generalized seizures. Brain Res 1986;397:377-80.
