Structural comparisons of meptazinol with opioid analgesics1
Introduction
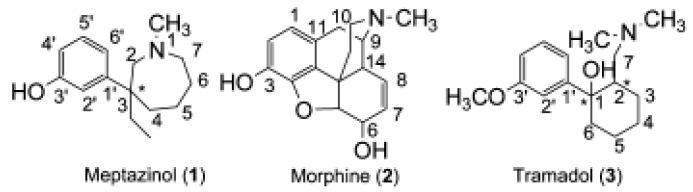
(±)-Meptazinol (Figure1), with one chiral center in the structure, is a potent analgesic similar to pethidine. Included in the British Pharmacopoeia in 1998[1], (±)-meptazinol was recommended by Hoskin and Hanks as one of six widely used agonist–antagonist analgesics[2]. However, to date its analgesic mechanism remains unknown. For example, we are still unsure if it is an agonist, an antagonist, or even a mixed agonist–antagonist, and whether it acts on one target or multiple targets.
During the early stages of development, meptazinol was regarded as a mixed agonist–antagonist opioid analgesic based on the following experimental evidence[3]: (i) the chemical structure of meptazinol is similar to that of morphine (Figure 1); (ii) meptazinol-induced analgesia is almost completely reversed by the opioid antagonist naloxone, although higher doses are required to reverse meptazinol compared to morphine; (iii) meptazinol is believed to be a selective μ opioid agonist[4]; and (iv) meptazinol reverses the signs of acute morphine overdose in animals and precipitates abstinence in animals rendered physically dependent on morphine.
However, unlike typical opiates whose notorious side-effects include respiratory depression and addiction, meptazinol induces little respiratory depression and has low addictive potential[5,6]. These properties make meptazinol quite similar to another analgesic, tramadol[7] (Figure 1), which is believed to have multiple analgesic mechanisms. Recent studies have also revealed that both tramadol and meptazinol differ from opioid analgesics in the lack of sensitization related to a low propensity to induce addition, which might greatly reduce the possibility of their abuse[8]. Furthermore, tramadol was found to induce its anti-nociceptive effects through monoamine neurotransmitters, whereas there was a cholinergic component in the action of meptazinol[9]. For example, the (–)-enantiomer of meptazinol was shown to be an inhibitor of acetylcholinesterase in vitro with potency 100-fold less than that of physostigmine[10]. All this evidence implies that there would be a unique analgesic mechanism for meptazinol.
Therefore, we are very interested in exploring the analgesic mechanism of meptazinol. Because the three-dimensional structures of opioid receptors are not available yet, as a first step of exploration we focused on structural comparisons of meptazinol with typical opiates and with tramadol. Our results will help elucidate the analgesic mechanism of meptazinol and will benefit the search for non-addictive analgesics.
Materials and methods
All calculations were carried out on a R14000 SGI Fuel workstation using the molecular modeling software package SYBYL version 6.9 (Tripos, St Louis, MO, USA).
Material preparation Both enantiomers of meptazinol were synthesized in our laboratory. Their analgesic activities were determined by observing the wrenching body reaction of mice after oral administration at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Their absolute configurations were determined using x-ray crystallography, co-crystallizing with dibenzoyl tartaric acid (DBTA), in our previous work[11], which was used as a starting point for this study. For each enantiomer of meptazinol, a random search was carried out on the seven-member ring to ensure a stable ring conformation. Then, all of the other rotatable bonds (except bonds within the seven-member ring) were selected for a systematic search with an interval of 30º for each bond to obtain the lowest energy conformation. After the initial simple energy minimization, the lowest energy conformation of meptazinol was further optimized using a quantum chemical calculation [semi-empirical Austin Model 1 (AM1) method[12] available in SYBYL]. This final optimized geometry of meptazinol was used in the subsequent structural comparisons.
Nine typical opiates, including agonists, antagonists and partial agonists, were selected to generate the opioid pharmacophore according to previous literature [13]. The initial atomic coordinates for seven of these opiates (ie morphine[14], 3-O-methyletorphine[15], azidomorphine[16], N-allynormetazocine[17], cyclazocine[18], naloxone[19], and nalbuphine[20]) were obtained from published x-ray crystallographic data and modeled using the CRYSIN tool of SYBYL. The other two opiates, codeine and 6-hydroxylevalorphan, were constructed based on their analogues, the crystal structures of which were available in SYBYL.
The initial structure of (R,R)-tramadol, the active isomer of tramadol, was retrieved from the MDL Drug Data Report-3D database (MDDR-3D), and underwent a similar optimizing treatment to meptazinol with one exception. All the rotatable bonds of tramadol (except bonds within the six-member ring) underwent a genetic algorithm conformational search to find the corresponding lowest energy conformation.
All structures were energy minimized for 1000 steps using the Tripos force field and POWELL method and the termination setting was 0.001 kcal/(mol×A).
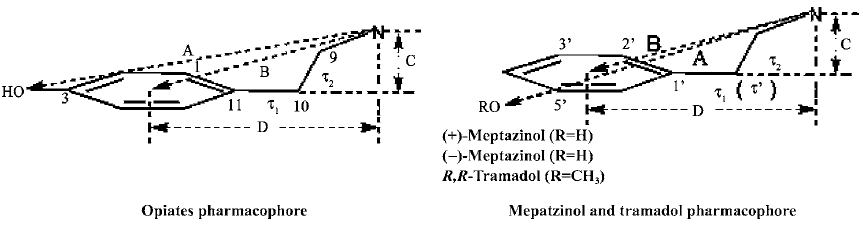
Analgesic pharmacophore generation The analgesic pharmacophore is defined in Figure 2. For comparison, related values were obtained from the literature[13] in which the pharmacophore was composed of four distances and two torsion angles. We extracted the common structures of nine opiates composed of a tyramine fragment considered to be the key pharmacophore according to the average values of the nine opiates. As shown in Figure 2, it should be noted that the hydroxyl group was located in the para-position of the phenyl ring in the opiates, but in the meta-position of the phenyl ring in meptazinol and tramadol (methoxyl instead of hydroxyl group in tramadol). Thus, we manually removed the two carbons between the nitrogen atom and the phenyl ring from the pharmacophore structure. The pharmacophore are composed of one protonated nitrogen atom and a phenol fragment. We superimposed all opiates to the pharmacophore template separately and calculated the average standard deviation value as shown in Table 1. In addition, we defined the pharmacophore for meptazinol and tramadol. One more torsion angle was defined in tramadol because the interval distance between the nitrogen atom and the phenyl ring is three carbon atoms in tramadol compared with two in the other compounds.
Full table
Structural superposition The method used to determine superposition was the database alignment facility in SYBYL, in which some or all of the molecules in the database were aligned with a template molecule also in the database. A common substructure was provided to evaluate the best “fit”. Only rigid-body rotations and translations were supported.
For the comparison of meptazinol with the opioid pharmacophore, the generated pharmacophore were set as the common substructure for alignment. Both enantiomers of meptazinol were superimposed on each other first, and then superimposed with the other opiates separately.
Using the same pharmacophore, both meptazinol enantiomers and tramadol were superimposed onto the template.
Results and Discussion
The analgesic activities were determined as ED50 values for the two enantiomers of meptazinol, 14.583 µmol/kg for (+)-enantiomer and 31.333 µmol/kg for the (–)-enantiomer. The (+)-enantiomer is more potent than the (–)-enantiomer, although the difference between them was not significant.
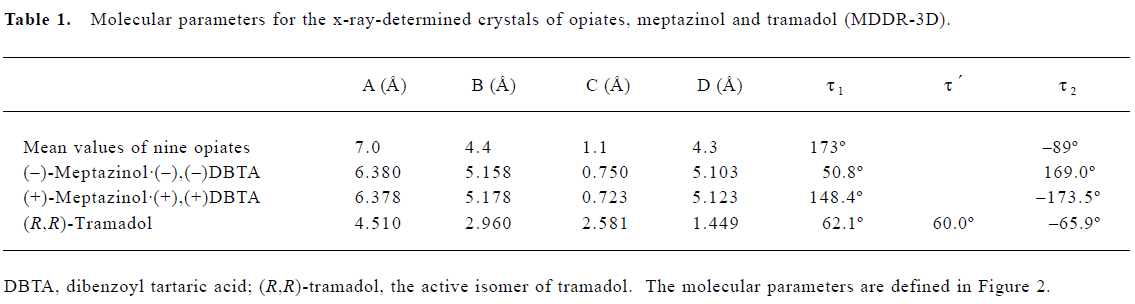
Because the structures of morphine and its derivatives and analogs are fairly rigid, it is reasonable to assume that their x-ray structures are the same as the active conformations binding to opioid receptors. Therefore, our pharma-cophore model was based on these rigid structures. According to the analgesic pharmacophore defined in Figure 2, we built this pharmacophore using the mean values of the nine opiates as the common substructure for superposition in SYBYL. For reasons mentioned above, we removed the two carbon atoms from the pharmacophore. We also measured the torsions of our pharmacophore structures without the removal of the carbon atoms to make our pharmacophore resemble the original one in the literature (Table 1).
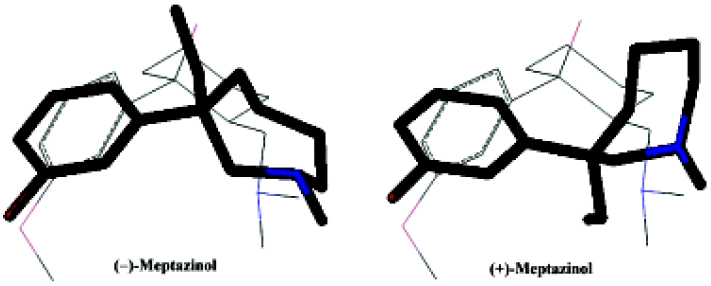
The crystal structures of the meptazinol enantiomers were treated with a series of methods to determine the lowest energy conformer for each enantiomer. According to the systematic search results for both meptazinol enantiomers, we found the lowest energy conformations to be very similar. The lowest energy conformation was selected as the low energy conformer followed by semi-empirical AM1 geometry optimization.
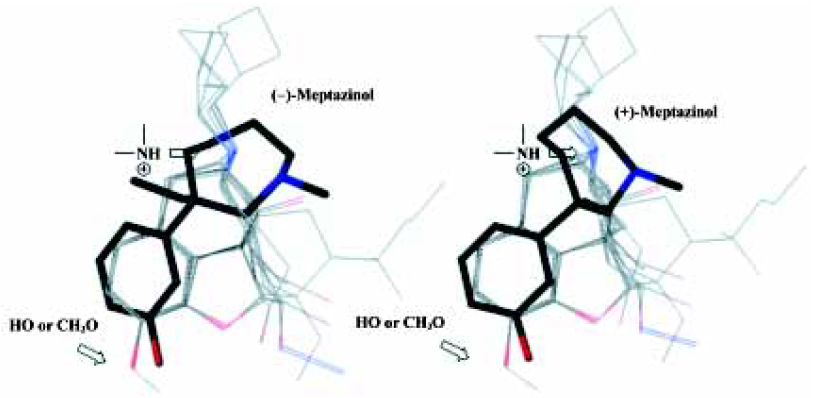
Using the opiate pharmacophore as the overlap template we first superimposed both enantiomers of meptazinol onto each other. As illustrated in Figure 3, the pharmacophore elements of the meptazinol enantiomers superimposed well onto each other. Thus, both meptazinol enantiomers may induce a similar analgesic mechanism, at least in part. An experiment using electrical stimulation of guinea pig isolated ileum indicated that both enantiomers of meptazinol were µ-selective opioid receptor agonists[21]. And the similar pharmacology results from observing the mice wrenching body reaction of meptazinol enantiomers also supports this conclusion, although for the (–)-enantiomer there is another cholinergic component in the participating antinociceptive effect[10].
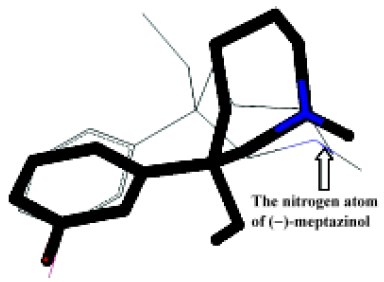
However, as demonstrated in Figure 4, the meptazinol pharmacophore differed from the opiate pharmacophore, particularly in the position of the nitrogen atom. The overlap of (+)-meptazinol to the opiate pharmacophore is a little better than (–)-meptazinol in the region of the phenol fragment, which may account for the minor increase in analgesic effect of (+)-meptazinol. These differences were also reflected in related molecular parameters. From Table 1, we can see that distance A in the pharmacophore of meptazinol was about 0.6 Å shorter than that in the opiates examined. The nitrogen atom actually becomes further away (>0.7 Å) from the phenol fragment in meptazinol than in the opiates, which was confirmed by the increased B and D values in meptazinol. Both enantiomers of meptazinol did not fit the skeletons of the opiates particularly well. The azepane ring of meptazinol did not match the corresponding alkyl chain between the nitrogen atom and the phenol fragment in the opiates (Figure 4). Whether or not the azepane ring contributes to analgesic potency needs further investigation.
In addition, we compared meptazinol with tramadol because they share more common pharmacological effects with each other than with other opiates. It should be noted that we still used the pharmacophore model generated from the opiate structures because their pharmacology may be mediated by a similar mechanism. To determine the active conformation of (R,R)-tramadol, a series of methods were carried out. Twenty-four minimum energy conformations of tramadol were analyzed and found to be very similar to each other. Therefore, the lowest energy conformation was selected and geometrically optimized further using the quantum chemical calculation method AM1, which resulted in the active conformation of tramadol. The related molecular parameters of tramadol are also listed in Table 1, and the superposition of tramadol with meptazinol is shown in Figure 5. Although the pharmacophore of meptazinol enantiomers and tramadol occupied a similar region, poor overlap occurred between meptazinol enantiomers and tramadol in the phenol fragment and the protonated nitrogen atom. Although (R,R)-tramadol shares some similar pharmacological effects with meptazinol, our studies revealed that their pharmacophores might be different.
Conclusion
In summary, from the structural comparisons conducted in the present study we learned that both enantiomers of meptazinol differed from typical opiates and from (R,R)-tramadol. However, the pharmacophore of both enantiomers of meptazinol might be similar to each other, and this result is consistent with previous studies[21]. Therefore, we suggest that meptazinol has a unique analgesic mechanism that is different from known analgesics (ie meptazinol may not target opioid receptors only, and one of its enantiomers might act on other targets in the cholinergic system). The antinociceptive targets and mechanism of meptazinol enantiomers needs to be investigated further.
Acknowledgements
We thank researchers from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences for the pharmacological test of the meptazinol enantiomers.
References
- Great Britain Medicines Commission. British Pharmacopoeia. London: Bernan Association; 1998. p 859.
- Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 1991;41:326-44.
- Green D. Current concepts concerning the mode of action of meptazinol as an analgesic. Postgrad Med J 1983;59 Suppl 1:9-12.
- Spiegel K, Pasternak GW. Meptazinol: a novel Mu-1 selective opioid analgesic. J Pharmacol Exp Ther 1984;228:414B.
- Goode PG, Rhodes KF, Waterfall JF. The analgesic and respiratory effects of meptazinol, morphine and pentazocine in the rat. J Pharm Pharmacol 1979;31:793-5.
- Johnson RE, Jasinski DR. Human pharmacology and abuse potential of meptazinol. Clin Pharmacol Ther 1987;41:426-33.
- Dayer P, Collart L. Pharmacology of tramadol. Drugs 1997;53 Suppl 2:18-24.
- Tzschentke TM, Bruckmann W, Friderichs E. Lack of sensitization during place conditioning in rats is consistent with the low abuse potential of tramadol. Neurosci Lett 2002;329:25-8.
- Holmes B, Ward A. Meptazinol. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy. Drugs 1985;30:285-312.
- Ennis C, Haroun F, Lattimer N. Can the effects of meptazinol on the guinea-pig isolated ileum be explained by inhibition of acetylcholinesterase? J Pharm Pharmacol 1986;38:24-7.
- Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP. AM1: a new general purpose quantum mechanical model. J Am Chem Soc 1985;107:3902-9.
- Horn AS, Rodgers JR. The enkephalins and opiates: structure-activity relations. J Pharm Pharmacol 1977;29:257-65.
- Mackay M, Hodgkin DC. A crystallographic examination of the structure of morphine. J Chem Soc 1955.3261.
- Van Den Hende JH, Nelson NR. The crystal and molecular structure of 7.alpha.-(1-(R)-hydroxy-1-methylbutyl)-6,14-endo-ethenotetrahydrothebaine hydrobromide (19-propylthevinol hydrobromide) J Am Chem Soc 1967;89:2901-5.
- Sasvari K, Simon K, Bognar R, Makleit S. The crystal and molecular structure of 6-deoxy-6-azido dihydroisomorphine, C17H20N4O2. Acta Cryst 1974;B30:634-41.
- Fedeli W, Giacomello G, Cerrini S, Vaciago A. Crystal and molecular structure of 2-allyl-2´-hydroxy-5,9-dimethyl-6,7-benzo-morphan mydrobromide monohydrate. J Chem Soc (B) 1970.1190-5.
- Karle IL, Girardi RD, Fratini AV, Karle J. The crystal structures of DL-cyclazocine and L-cycla-zocine.HBr.H2O, and the absolute configuration of L-cyclazocine.HBr.H2O (2-cyclo-propyl-methyl-2´-hydroxy-5,9-dimethyl-6,7-benzomorphan). Acta Cryst 1969;B25:146979.
- Sime RL, Forehand R, Sime RJ. The crystal structure of a narcotic antagonist: naloxone hydrochloride dehydrate. Acta Cryst 1975;B31:2326-30.
- Sime RJ, Dobler M, Sime RL. The crystal structure of a narcotic agonist/antagonist: nalbuphine hydrochloride dehydrate. Acta Cryst 1976;B32:809-12.
- Duchesne RJ, Goodall J, Hughes IE. Pharmacological effects of meptazinol and its enantiomers on guinea-pig ileum and mouse vas deferens. J Pharm Pharmacol 1984;36:560-2.