Triptolide suppresses CD80 and CD86 expressions and IL-12 production in THP-1 cells1
Introduction
Human cells of monocytic lineage, including monocytes, macrophages, and monocyte-derived dendritic cells, play an essential role in initiating and maintaining immune responses by acting as antigen-presenting cells (APC). They process and present antigenic peptides via the major histocompatibility complex (MHC) molecules and the expression of costimulatory molecules[1,2]. The best-characterized costimulatory molecules to date are two structurally related proteins, CD80 and CD86. Both provide costimulation to T-cells, leading to their proliferation, cytokine production, and development[2,3]. In vivo studies using anti-CD80 and anti-CD86 monoclonal antibodies or genetically manipulated animals have demonstrated that a blockade of CD80/CD86 costimulatory molecules can prolong allograft survival and reduce the severity of autoimmune diseases[4,5]. Preliminary clinical studies in human subjects also support this concept[6]. Therefore, pharmacological intervention in the upregulation of CD80/CD86 may therefore be useful in the treatment of autoimmune diseases or in transplantation.
Tripterygium wilfordii Hook F (TWHF) is a herb used in traditional Chinese medicine for the treatment of rheumatoid arthritis and other autoimmune diseases[7,8]. Triptolide, a highly oxygenated diterpenoid triepoxide, is the major component responsible for the immunosuppressive and anti-inflammatory effects of TWHF[9]. It is effective for the treatment of autoimmune diseases, prevention of allograft rejection, and graft-versus-host disease in animal experiments[10–13]. The inhibitory effect of triptolide on T-cell activation is stronger than cyclosporine[14] and FK-506[15] in vitro. Furthermore, it can induce activated T-cell apoptosis[16]. Thus, T-cells are considered to be the main target for triptolide. Recently, Hong et al[17] and Li et al[18] demonstrated that triptolide inhibited upregulation of B7 on activated human proximal tubular epithelial cells. These results suggest that triptolide may have suppressive effects on APC. In a further analysis of this theory, we used a human monocytic leukemia cell line (THP-1) that had been differentiated into macrophage-like cells by treatment with Me2SO. The macrophage-like THP-1 cells were used to examine the influences of triptolide on the expression of CD80 and CD86 molecules and the production of IL-12, an important cytokine that biases CD4+ T-cells toward Th1 differentiation[19].
Materials and methods
Reagents Triptolide was provided by the Department of Chemistry, Shanghai Institute of Materia Medica. Lipopolysaccharide (LPS, Escherichia coli 055: B5), 3-[4,5-dimethylthylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT), and 3,3´,5,5´-tetramethylbenzidine (TMB) were from Sigma (St Louis, MO, USA). Recombined human IFN-γ was purchased from Shanghai Clonbiotech (Shanghai, China). PE-conjugated mAbs were used to detect cell surface expression of CD14, HLA-ABC, HLA-DR, CD80 and CD86 by flow cytometry, as well as isotype-matched mAbs,which were purchased from PharMingen (San Jose, CA, USA).
Cells The human monocytic THP-1 line (ATCC, Manassas, VA, USA) was maintained in suspension culture in RPMI1640 medium, supplemented with 10% FBS. Cultures were maintained at 37 oC in a humidified atmosphere of 5% CO2 and were subcultured at 1/10 dilution every 5–6 d. Cells were used to differentiate when seeded at a density of 5×108 cells/L in a 10-cm2 culture dish for 24 h.
Flow cytometry analysis THP-1 cells were cultured in a screw cap container 2.4×106 cells in 4 mL of RPMI1640 medium with 10% FBS per-well. Cells were differentiated with 1.2% Me2SO for 24 h, then pre-treated with IFN-γ (500 kU/L) for 16 h and stimulated by LPS (1 mg/L) for another 24 h. Various concentrations of triptolide were added at the initiation of the culture. Cells were collected and washed in an ice cold staining buffer (PBS containing 0.1% w/v sodium azide, 1% FBS, pH 7.2). Cells were then resuspended at 4.0–5.0×109/L in an ice-cold staining buffer. Optimal concentrations of each fluorochrome-labeled antibody were added. Cells were incubated at 4 oC for 30 min, then washed twice and analyzed by flow cytometry using FACSCalibur (Becton Dickinson, San Jose, CA,USA). Data were illustrated by CELLQuest.
Cytokine analysis THP-1 cells were cultured in 24-well plates at 6.0×105 cells in 2 mL of RPMI1640 medium with 10% FBS and other treatments were the same as in the surface molecules analysis. Culture supernatants were harvested and stored at -20 oC. Human IL-12p70 and IL-12p40 productions were measured using ELISA kits (PharMingen) according to the manufacturer’s instructions.
MTT assay Cytotoxicity was assessed by MTT assay[20]. Me2SO-differentiated THP-1 cells were incubated in a 96-well plate at 5.4×104 cells in 180 µL of RPMI1640 medium with 10% FBS per-well for 72 h with and without various concentrations of triptolide. MTT (5 g/L) 18 µL was added 5 h prior to the end of the culture, and then 90 µL solvent (10% SDS, 50% N, N-dimethy formamide, pH 7.2) was added. The cells were then incubated for another 7 h and value was measured at 570 nm by a microplate reader (Bio-Rad Model 550, Japan).
Statistical analysis Results were expressed as mean±SD. An independent two-tailed t-test was performed and P<0.05 was considered to be statistically significant.
Results
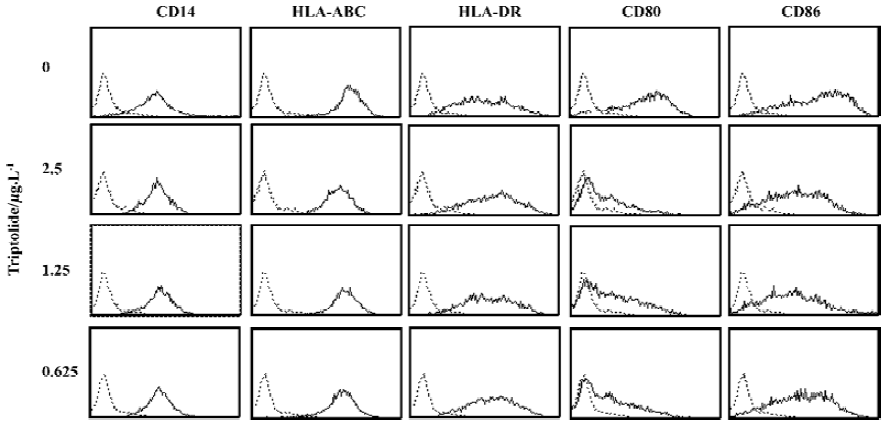
Triptolide influenced the surface molecule expressions on Me2SO-differentiated THP-1 cells THP-1 cells are known to be activated by LPS stimulation and have been used as a surrogate for investigation of human monocytes[21]. When THP-1 cells are treated with Me2SO, the cells differentiate into macrophage-like cells and proliferation is profoundly inhibited. IFN-γ pretreatment and LPS stimulation enhanced the expression of a variety of surface molecules such as HLA-ABC, HLA-DR, and B7 molecules on THP-1 cells. Tripolide could suppress CD80 and CD86 expressions on IFN-γ (500 kU/L) and LPS (1 mg/L)-activated THP-1 cells at 2.5–0.625 µg/L (Figure 1). Triptolide also weakly down-regulated the HLA-ABC expression, but it did not change the expressions of HLA-DR and CD14 on THP-1 cells.
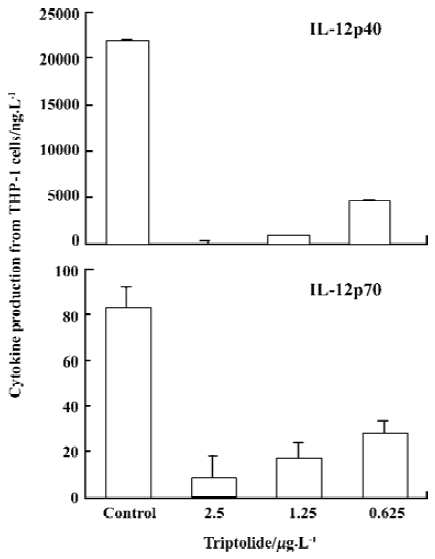
Triptolide inhibited IL-12 production of Me2SO-differentiated THP-1 cells IFN-γ pretreatment and LPS stimulation induced IL-12p40 and IL-12p70 production from Me2SO-differntiated THP-1 cells. The productions of IL-12p40 and IL-12p70 in response to IFN-γ (500 kU/L) and LPS (1 mg/L) induction were significantly reduced in THP-1 cells exposed to triptolide in the concentration of 2.5–0.625 µg/L (Figure 2). IL-10 production was too low to be detected in this experiment system.
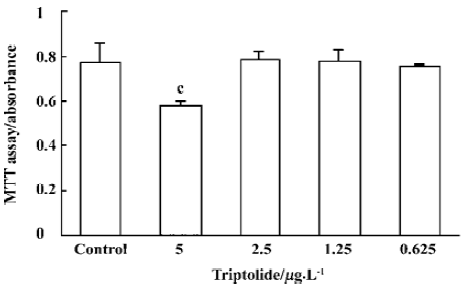
Cytotoxicity of triptolide on Me2SO-differentiated THP-1 cells We examined whether the above suppressive effects of triptolide were due to its cytotoxicity to THP-1 cells. When Me2SO-differentiated THP-1 cells were cultured with and without different concentrations of triptolide for 72 h, the cytotoxicity was monitored by MTT assay. As shown in Figure 3, triptolide did not exert any cytotoxic effect on the THP-1 cells from 2.5 µg/L to 0.625 µg/L in the present study.
Discussion
The immunosuppressive and therapeutic activity of triptolide upon systemic administration has been demonstrated in animal models for human-like diseases, such as in experimental autoimmune uveitis[22], collagen-induced arthritis[11], diabetes[12], nephrotic syndrome[13]. It also improves the survival rate of the heart[23] and kidney[24] allograft in the rat transplantation model and graft-versus-host disease in a murine allogeneic bone marrow transplantation model[25]. However the immunosuppressive mechanism of triptolide has not been completely delineated[26]. In the present study, we demonstrated a new finding that triptolide, an active component of TWHF, directly down-regulats the costimulation molecule CD80 and CD86 expressions on macrophages-like cells (Me2SO-differentiated THP-1 cells), and inhibits the bioactive form of IL-12p70 production from Me2SO-differentiated THP-1 cells by a dose-dependent manner.
CD80 and CD86 on APC delivered the critical costimulatory signals for optimal T-cell activation[3]. Blockade of CD80 and CD86 signaling resulted in ineffective T- cell activation. The implication is that reduced and blocked CD80/CD86 costimulations can prevent the induction of pathogenic T-cell responses in autoimmune disease and allow for prolonged acceptance of allografts in organ transplantation[4–6]. In addition to the processing and presentation of antigenic peptides via MHC molecules and the expression of costimulatory molecules, APC played a central role in driving CD4+ T helper cell differentiation through the production of critical cytokines, such as IL-12, IL-10, TGF-β and possibly IL-4. The production of bioactive form IL-12p70 by host antigen-presenting cells was a primary determinant in the differentiation of naive CD4+ T cells into Th1 effector cells[19]. These suppressive effects of triptolide on CD80 and CD86 expressions, and IL12p70 production suggest an important therapeutic implication for multiple sclerosis and other abnormal immune response mediated diseases associated with the increased expression of CD80/CD86 and the production of IL-12[27].
Triptolide has been proven to be a potent immunosuppressive agent, although information on the molecular mechanism of triptolide is scarce. Recent studies have shown that triptolide inhibits T-cell activation and early cytokine gene transcription in T-cell at the purine-box/ARRE/NF-AT and NF-κB target sequence after specific DNA binding[28,29]. NF-κB is a family of transcription factors central to immunity and inflammation. It regulates expression of a number of genes, including cytokines, costimulatory molecules, nitric oxide, and susceptibility to apoptosis[30]. Therefore, the suppressive effects of triptolide on the co-stimulatory molecular expression and IL-12 production from THP-1 cells can result from the inhibition of NF-κB transcriptional activation.
In conclusion, we have demonstrated that triptolide down-regulats the levels of CD80 and CD86 expressions in THP-1 cells. Production of bioactive form IL-12p70 in THP-1 cells was effectively inhibited by triptolide. These immunosuppressive activities of triptolide contribute, at least in part, to the therapeutic effect and mechanism of triptolide in the treatment of autoimmune diseases.
Footnote
Project supported by the National Natural Science Foundation of China (N
References
- Schwartz RH. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol 1985;3:237-61.
- Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation. Crit Rev Immunol 1998;18:389-418.
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 2001;19:225-52.
- Jonker M, Ossevoort And MA, Vierboom M. Blocking the CD80 and CD86 costimulation molecules: lessons to be learned from animal models. Transplantation 2002;73:S23-6.
- Anand S, Chen L. Control of autoimmune diseases by the B7-CD28 family molecules. Curr Pharm Des 2004;10:121-8.
- Gottlieb AB, Lebwohl M, Totoritis MC, Abdulghani AA, Shuey SR, Romano P, et al. Clinical and histologic response to single-dose treatment of moderate to severe psoriasis with an anti-CD80 monoclonal antibody. J Am Acad Dermatol 2002;47:692-700.
- Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, et al. A pros-pective, controlled, double-blind, cross-over study of tripterygium wilfodii hook F in treatment of rheumatoid arthritis. Chin Med J 1989;102:327-32.
- Lipsky PE, Tao XL. A potential new treatment for rheumatoid arthritis: thunder god vine. Semin Arthritis Rheum 1997;26:713-23.
- Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook f. Drugs R D 2003;4:1-18.
- Su D, Song Y, Li R. Comparative clinical study of rheumatoid arthritis treated by triptolide and an ethyl acetate extract of Tripterygium wilfordii. Chin J Integrated Trad West Med 1990;10:144-6.
- Gu WZ, Brandwein SR. Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol 1998;20:389-400.
- Li L, Wakukami T, Teng WP, Di GX, Murakami K, Mimura G. The effect of triptolide on the development of insulitis in low-dose streptozotocin induced diabetic mice and on the beta-cells of normal mice. Chin J Diabetes 1994;2:69-75.
- Yi ZW, Li L. Clinical study of nephrotic syndrome in children treated by triptolide. Hunan Med J 1994;11:284-5.
- Lu H, Hachida M, Enosawa S, Li XK, Suzuki S, Koyanagi H. Immunosuppressive effect of triptolide in vitro. Transplant Proc 1999;31:2056-7.
- Chan MA, Kohlmeier JE, Branden M, Jung M, Benedict SH. Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. Phytother Res 1999;13:464-7.
- Yang Y, Liu Z, Tolosa E, Yang J, Li L. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology 1998;40:139-49.
- Hong Y, Zhou W, Li K, Sacks SH. Triptolide is a potent suppressant of C3, CD40 and B7h expression in activated human proximal tubular epithelial cells. Kidney Int 2002;62:1291-300.
- Li H, Liu ZH, Dai CS, Liu D, Li LS. Triptolide inhibits proinflam-matory factor-induced over-expression of class II MHC and B7 molecules in renal tubular epithelial cells. Acta Pharmacol Sin 2002;23:775-81.
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 1998;16:495-521.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Method 1983;65:55-63.
- Kubin M, Chow JM, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor alpha, and IL-1 beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood 1994;83:1847-55.
- Wu Y, Wang Y, Zhong C, Li Y, Li X, Sun B. The suppressive effect of triptolide on experimental autoimmune uveoretinitis by down-regulating Th1-type response. Int Immunopharmacol 2003;3:1457-65.
- Lin JF, Zhu H, Liu XQ, Zhen HP, Zheng YL. Triptolide prolong the survival of heart transplant in mice. Strait Pharmacentical J 1998;10:29-30.
- Yang J, Fan Z, Dai C, Wang J, Liu Z, Li L. Antirenal allograft rejection effects of triptolide in rats. J Nephrol Dialy Transplant 1997;6:214-20.
- Chen BJ, Liu C, Cui X, Fidler JM, Chao NJ. Prevention of graft-versus-host disease by a novel immunosuppressant, PG490-88, through inhibition of alloreactive T cell expansion. Transplantation 2000;70:1442-7.
- Du ZY, Li XY, Li YC, Wang SY. Analysis of triptolide-regulated gene expression in Jurkat cells by complementary DNA micro-array. Acta Pharmacol Sin 2003;24:864-72.
- Monteyne P, Guillaume B, Sindic CJ. B7-1 (CD80), B7-2 (CD86), interleukin-12 and transforming growth factor-beta mRNA expression in CSF and peripheral blood mononuclear cells from multiple sclerosis patients. J Neuroimmunol 1998;91:198-203.
- Liu H, Liu ZH, Chen ZH, Yang JW, Li LS. Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Acta Pharmacol Sin 2000;21:782-6.
- Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem 1999;274:13443-50.
- Caamano J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev 2002;15:414-29.
