Intrathecal administration of roscovitine inhibits Cdk5 activity and attenuates formalin-induced nociceptive response in rats1
Introduction
Tissue injury is associated with sensitization of nociceptors and subsequent changes in the excitability of central neurons, known as central sensitization. Nociceptor sensitization and central sensitization are believed to underlie the development of primary and secondary hyperalgesia[1]. Glutamate, acting at the spinal N-methyl-D-asparate (NMDA) receptor, has been implicated in the development of secondary hyperalgesia[2,3]. Downstream of NMDA receptor activation, spinal nitric oxide (NO), protein kinase C, and other mediators have been implicated in maintaining such hyperalgesia[4]. Among these mediators, cyclin-dependent kinase-5 (Cdk5) has been found to be involved in modulation of the NMDA and metabotropic glutamate receptors[5].
DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa) is a cytosolic protein that is selectively enriched in medium spiny neurons in the neostria-tum[6]. When DARPP-32 is phosphorylated by cAMP-dependent protein kinase (PKA) on Thr34, it is converted into a potent inhibitor of protein phosphatase-1 (PP-1)[7]. This leads to an increase in the phosphorylation of downstream PP-1 substrates, including various neurotransmitter receptors and voltage-gated ion channels[6]. If DARPP-32 is phosphorylated at Thr75 by Cdk5, it inhibits PKA activity and thereby reduces the efficacy of dopamine signaling[8]. Thus, DARPP-32 is a bi-functional signal transduction molecule that controls the activity of PP-1 and PKA by phosphorylation at different sites.
Roscovitine is a potent selective inhibitor of Cdk5, and competes for the ATP-binding site of the kinase (ED50=0.16 µmol/L)[9]. Injection of roscovitine inhibits Cdk5 activity in the hippocampus and reduce the NMDA-evoked currents[10]. Recently, we demonstrated roscovitine mediated antinocicep-tion and attenuated morphine tolerance in rats[11]. Therefore, in the present study, the effect of roscovitine on the nociceptive flinch response, evoked by subcutaneous formalin injection, and its possible molecular mechanism, was investigated.
Materials and methods
DrugRoscovitine was obtained from LC Laboratories (Woburn, MA, US) and Me2SO was purchased from Sigma-Aldrich (St Louis, MO, USA).
Animal care and intrathecal catherterizationMale Sprague-Dawley rats (250–300 g) were provided by the “National” Science Council, Taiwan, China. The rats were housed in a room with a 12:12 h dark-light cycle, and a temperature of 22±0.5 ºC, with food and water ad libitum. The ethical guidelines specified by the Chang-Gung Memorial Hospital Animal Ethics Committee were followed throughout the study. Chronic intrathecal catheters were implanted under isoflurane anesthesia. Through an incision in the atlanto-occipital membrane, a polyethylene (PE-5) catheter, filled with 0.9% saline, was advanced 8.5 cm caudally to position its tips at the level of the lumbar enlargement. The rostral tip of the catheter was passed subcutaneously, externalized on top of the skull, and sealed with a stainless-steel plug. Rats showing neurological deficits after implantation were excluded from the study. Rats were used for experimentation three days after implantation.
Behavioral testing and animal groupingFor formalin injection, 50 µL of a 5% formalin solution was injected subcutaneously into the dorsal surface of the right hind paw using a 27-gauge needle. Animals were then placed in a clear Plexiglas cylinder (30 cm×30 cm) for observation. A mirror was placed below the floor (Plexiglas) at a 45º angle, to enable unencumbered observation during the test. Within 1 min of the injection, the rats displayed the typical behavior of this model, holding the paw just off the floor. During this period, spontaneous flinching of the injected paw was also observed. Flinching was readily discriminated, and was characterized as a rapid and brief withdrawing or flexing of the injected paw. Pain-related behavior was quantified by counting the number of flinches over 1 min intervals during the first 5 min, and then at 5 min intervals 10–60 min after formalin injection. Two phases of spontaneous flinching responses were observed. An initial acute pain response (phase I, during the first 1–5 min after formalin injection) was followed by a relatively short quiescent period and then by a prolonged tonic phase (phase II, beginning approximately 10–60 min after formalin injection). Criteria for exclusion from the study included incomplete formalin injection, or excessive bleeding from the injection site. Time-response data was presented as the mean±SD per minute for the period of 1–9 min, and then at 5 min intervals up to 60 min. For the dose-response analysis, data from phase I and phase II observations were considered separately. In each case, the observation interval was calculated for each rat. The cumulative flinching response was calculated for each animal, and the dose-response curve represents the mean±SD. To determine the dose dependency and time course of the antinociceptive action of an intrathecal injection of roscovitine, animals were randomly assigned to five groups receiving different doses of roscovitine: 0, 10, 50, 100, 200 µg (n=6 in each group), injected intrathecally 30 min before formalin administration. For exploring possible µ-opioid involvement in the effect of intrathecal roscovitine, naloxone (1 mg/kg) was given intraperitoneally 1 h before the formalin test in the 200 µg roscovitine group (n=6). Roscovitine was dissolved in dimethyl sulfoxide (Me2SO) and delivered with a microsyringe in a total volume of 10 µL, followed immediately by 5 µL of Me2SO to flush out the catheter.
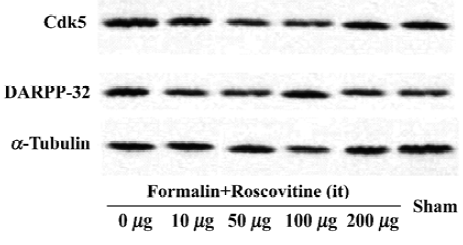
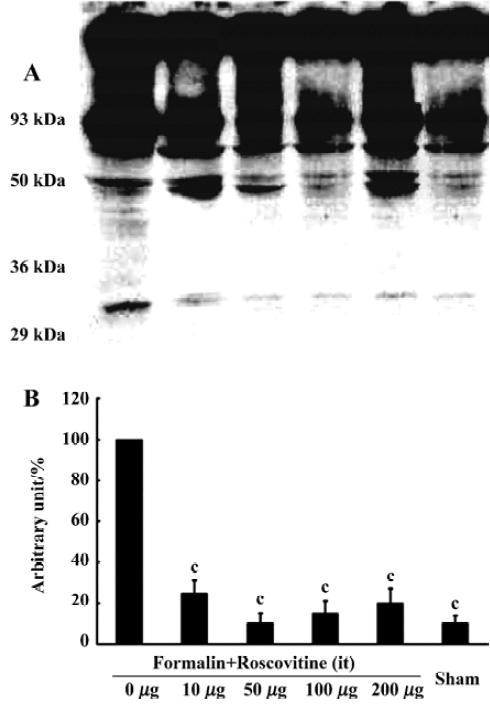
Western blot analysis One hour after formalin injection, the rats were killed under deep isoflurane anesthesia, then decollated and spinal cord was removed. The lumbar spinal cords were homogenized in a lysis buffer (20 mmol/L Tris pH 7.6, 150 mmol/L NaCl, 1 mmol/L EGTA, 5 mmol/L NaF and 1 mmol/L dTT, supplemented with protease inhibitor cocktail tablets (Roche, Mannheim, Germany) and complete phosphatase inhibitors. For analysis of Cdk5 and DARPP-32 protein expression after the formalin test, 25 µg protein extracts were electrophoresed on a 12% acrylamide SDS polyacrylamide gel electrophoresis and immunoblotted onto polyvinylidene fluoride membranes. The membranes were blocked for 1 h at room temperature and incubated overnight with Cdk5 (C-8) (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), DARPP-32 (1:1000) (Cell Signaling Technology, Beverly, MA, USA), phospho-DARPP-32 (Thr-75) antibody (1:500) (Cell Signaling Technology), and α-tubulin antibody (1:1000) (Santa Cruz Biotechnology) was used as an internal control. Antibody binding was detected using a goat anti-rabbit horseradish peroxidase-linked IgG. The bands were visualized by an ECL detection system (Amersham-Pharmacia Biotech, Little Chalfont, England). Band intensities were quantified by using an image analyzer (Densitograph AE-6900M, Atto, Tokyo, Japan).
Statistical analysisAll the data in this study are presented as means±SD and analyzed by one-way ANOVA followed by Dunnett’s test for post-hoc analysis. P<0.05 was considered significant.
Results
Roscovitine attenuated the formalin-induced flinch responseFormalin (5%, 50 µL) injected into the dorsal surface of a rat hind paw produced characteristic behaviors. The intrathecal administration of 0, 10, 50, 100, and 200 µg roscovitine produced a dose-dependent decrease in the flinch response to formalin (Figure 1). The cumulative phase I flinch counts were 79±6, 81±4, 60±7, 60±6, and 23±2, respectively. There was a statistically significant difference between the 200 µg group and the 0 µg group (P<0.01). The cumulative phase II flinch counts were 117±6, 112±7, 65±25, 46±11, and 16±2. The 50, 100, and 200 µg groups all showed significant inhibition of the phase II flinch response compared to the 0 µg group (P<0.01).

Naloxone reversed the roscovitine mediated anti-nociception Another six rats were given naloxone (1 mg/kg) 1 h before the formalin test in the 200 µg roscovitine group. The cumulative phase I flinch count was 48±5 and the phase II flinch counting was 92±10. The difference in phases I and II were statistically significant compared to the 200 µg group (P<0.01) (Figure 1).
Roscovitine attenuated the phosphorylated DARPP-32 upregulation after formalin hyperalgesiaWestern blot analysis showed that the Cdk5 expression did not change significantly in any groups, and neither did the total DARPP-32, the downstream target of Cdk5 (Figure 2). However, the phosphorylated-DARPP-32 at Thr-75 was upregulated significantly after formalin hyperalgesia compared to the sham group, which did not receive formalin injection on the paw. Intrathecal roscovitine attenuated the increase in the proportion of phosphorylated DARPP-32 proportion significantly as compared to the 0 µg group [25±6, 10±5, 15±6, 20±7, and 10±4 (arbitrary percentage calculated by phosphorylated-DARPP-32 Thr-75/DARPP-32) in 10 µg, 50 µg, 100 µg, 200 µg, and sham groups respectively after three separate experiments, P<0.01] (Figure 3).
Discussion
The formalin test is a model that is believed to underlie abnormal pain perception in humans following injury[12,13]. Following subcutaneous injection of formalin into the hind paw of a rat, the animal displays spontaneous pain behavior, that is, increased hind paw flinching and licking[14]. Two distinct phases were observed, an early acute phase (phase I) followed by a later tonic phase (phase II). The response to formalin during the acute phase is attributed to the pain intensity itself. The response during tonic phase is believed to be mediated through increased spontaneous activity of the spinal cord dorsal horn neurons[15].
Cdk5 is a member of the Cdk family of serine/threonine kinases. Recently, both NMDA and metabotropic glutamate receptors have been shown to be modulated by Cdk5[5]. Previous experiments have shown that inhibition of Cdk5 activity in hippocampal CA1 neurons by roscovitine injection results in a reduction of long-term potentiation and NMDA-evoked currents[10]. Cdk5 may be one of the most important kinases in the regulation of neurotransmitter release. The induction of neurotransmitter release by Cdk5 inhibitors is caused by the regulation of P/Q-type voltage-dependent calcium channel activity[16]. Therefore, Cdk5 might play an important role in nociception by controlling neurotransmitter release.
In this study, intrathecal roscovitine reduced the flinch response after formalin hyperalgesia in a dose-dependen manner. Western blot analysis revealed that the levels of phosphorylated DARPP-32 at Thr-75 were increased after formalin hyperalgesia, and this upregulation was blocked by intrathecal roscovitine administration. Hence, Cdk5 may affect this inflammatory pain model through the DARPP-32 pathway.
Interestingly, naloxone markedly decreased intrathecal roscovitine-induced antinociception, suggesting the involvement of µ-opioid receptors. It is noteworthy that naloxone did not completely block roscovitine-induced antinocicep-tion. The exact underlying mechanism is not clear. However, it might suggest that the µ-opioid receptor and Cdk5 are integrated in the DARPP-32 pathway as reported by Green-gard[6].
This is the first study exploring the antinociceptive effect of intrathecal roscovitine on formalin-induced pain. Our findings suggest an induction of phosphorylated-DARPP-32 at Thr-75 after formalin hyperalgesia. Intrathecal roscovi-tine administration attenuated these flinch responses, at least in part, through inhibition of Cdk5 activity. Taken together, these data suggest that spinal Cdk5 activity might play an important role in nociception, and that its inhibitor, roscovi-tine, could be applied in the management of acute inflammatory pain.
Footnote
This work was supported by research grants from the Chang Gung Memorial Hospital (CMRPG- 83027, 83030, and 83033).
References
- Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci USA 1999;96:7680-6.
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA 1999;96:7687-92.
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H. Involvement of spinal N-methyl-D-aspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci Lett 2001;316:83-6.
- Dolan S, Nolan AM. N-methyl-D-aspartate induced mechanical allodynia is blocked by nitric oxide synthase and cyclooxygenase-2 inhibitors. Neuroreport 1999;10:449-52.
- Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, et al. Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. J Neurochem 2002;81:832-41.
- Greengard P. The neurobiology of slow synaptic transmission. Science 2001;294:1024-30.
- Hemmings HC, Nairn AC, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phospho-protein. II. Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor 1. J Biol Chem 1984;259:14491-7.
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature 1999;402:669-71.
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997;243:527-36.
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA 2001;98:12742-7.
- Wang CH, Lee TH, Tsai YJ, Liu JK, Chen YJ, Yang LC, et al. Intrathecal cdk5 inhibitor, roscovitine, attenuates morphine antinociceptive tolerance in rats. Acta Pharmacol Sin 2004;25:1027-30.
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 1992;263:136-46.
- Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res 1990;535:155-8.
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain 1992;51:5-17.
- Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurons in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain 1987;30:349-60.
- Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K, et al. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci 2002;22:2590-7.
