Antiapoptotic effect both in vivo and in vitro of A20 gene when transfected into rat hippocampal neurons1
Introduction
Uncontrolled gene expression and apoptosis result in the development of various diseases. Excessive apoptosis plays a role in the pathogenesis of AIDS and neurological diseases such as Alzheimer disease and stroke[1].
The zinc finger protein A20 is a novel protein type in that it seems to have a dual activity, both as an inhibitor of NF-κB activation and as an antiapoptotic molecule in some cell systems, but not in the case of human cervix carcinoma HeLa cells, lung epithelial A549 cell, or human hepatoma HepG2 cells[2–6]. The reason why some cell lines are protected by A20 and others not are still unclear.
Little research work has been done on the functions of A20 in primary cultured neurons, although cell apoptosis is involved in many neurological diseases. The poor transfection efficiency resulting from the extreme resistance of cultured neurons to chemical transfection vectors has limited further research[7].
In this study, we overexpressed A20 by means of electro-poration gene transfer into neurons to investigate whether A20 can inhibit TNF-induced apoptosis in primary cultured neurons both in vivo and in vitro.
Materials and methods
Cloning of the A20 gene Human umbilical vein endothelial cells were treated with TNF-α 1000 kU/L and cycloheximide 10 µg/L for 4 h. Poly(A)+-mRNA was extracted with Trizol Reagents (Gibco) and identified by electrophoresis on a 1.2% agarose gel. cDNA was obtained by using reverse transcription-polymerase chain reaction (PCR). The PCR was performed with a sense primer (5´-AAC GAGCG-GTTCCGATGCC-CTGAG-3’) and an anti-sense primer (5´-TGTCGCCTTCACCGTTC-CAGTT-3´) using 30 cycles of 95 ºC for 1 min, 62 ºC for 1.5 min, 72 ºC for 2.5 min. The pcDNA3 expression vector containing the CMV immediate early promoter was used for this study.
Analysis of A20 mRNA contentsIsolation of total mRNA from the transfected neurons cultured in vitro or in the penumbra tissue was performed using Trizol reagent (Promega, Charbonnieres, France), followed by RT-PCR using the following primers: forward 5´-CGGTACCGCACA-ATGGCTGAACAAGTCCTTCCT-3´ and reverse 5´-CGTC-TAGAGTTAGCCATACATC-TGCTTGAACTG-3´. The PCR was run at 95 ºC for 5 min, followed by 30 cycles of 95 ºC for 1 min, 62 ºC for 1.5 min, and 72 ºC for 2.5 min, with a 10 min final extension period at 72 ºC. The housekeeping gene β-actin was used as a control.
Western blotting Protein was isolated from the transfected neurons and the penumbra of the MCAO rats using RIPA reagent 10 d after transfection. The samples were then resolved on a 15% SDS-PAGE gel and electrophoretically transferred onto poly-vinylidene difluoride membranes. The membranes were probed overnight at 4 ºC with mouse monoclonal anti-human A20 antibody (1/2000, Oncogene). After being incubated with goat anti-mouse-HRP (1/5000) for 1 h at room temperature, antigens were revealed by enhanced chemilumincescence reaction buffer.
Cell cultureHippocampal neurons from embryonic day 18 were freshly prepared by hippocampal dissection. The C-shaped hippocampus was cut off and trypsinized for 20 min at 37 ºC in a 5% CO2 incubator. The cells were broken apart by pipetting with complete media and were then plated with a density of 3×108 cells/L in neurobasal medium supplemented with B27 (all materials from Gibco Invitrogen, Grand Island, NY).
Rat neuron Nucleofector transfectionTransfection was accomplished using a Rat Neuron Nucleofector kit and the device from Amaxa. For each example, the prepared neurons were resuspended in Rat Neuron Nucleofector Solution to a final concentration of 1×106–6×106 cells/L. Then, 100 µL of cell suspension was mixed with 1–3 µg DNA (in 1–5 µL H2O or TE). Each nucleofection sample was transferred into an Amaxa certified cuvette. Insert the cuvette into the cuvette holder (Amaxa, Germany) and rotate the turning wheel clockwise to the final position. And tranfection program G-13 was initiated. The transfections efficiencies were evaluated and cell transplantation was carried out 3 d later.
ImmunocytochemistryThree days after transfection, the wells were fixed with 4% paraformaldehyde for 15 min. Then the wells were incubated for 12 h at 4 ºC with mouse anti-rat NF antibody (1:100, DAKO) and mouse anti-human A20 antibody (1:200, Oncogene), diluted in blocking buffer. The wells were incubated with rhodamine-labeled secondary antibodies (1/500, Kirkegaard Perry Laboratories, MD, USA) and FITC-labeled secondary antibodies (1/200, DAKO) in blocking buffer for 60 min at room temperature. Fluorescence staining was evaluated using a Leica fluorescent microscope.
Cell counting Neurons stained positively for A20 were counted on coded slides using an Olympus CAST Grid system (Denmark). The area of each culture well was delineated and a counting frame was randomly placed to mark the first area to be sampled. The frame was then systematically moved through the delineated area. The total number of neurons and A20-immunoreactive neurons was extrapolated from the data, and the transfection efficiency was evaluated.
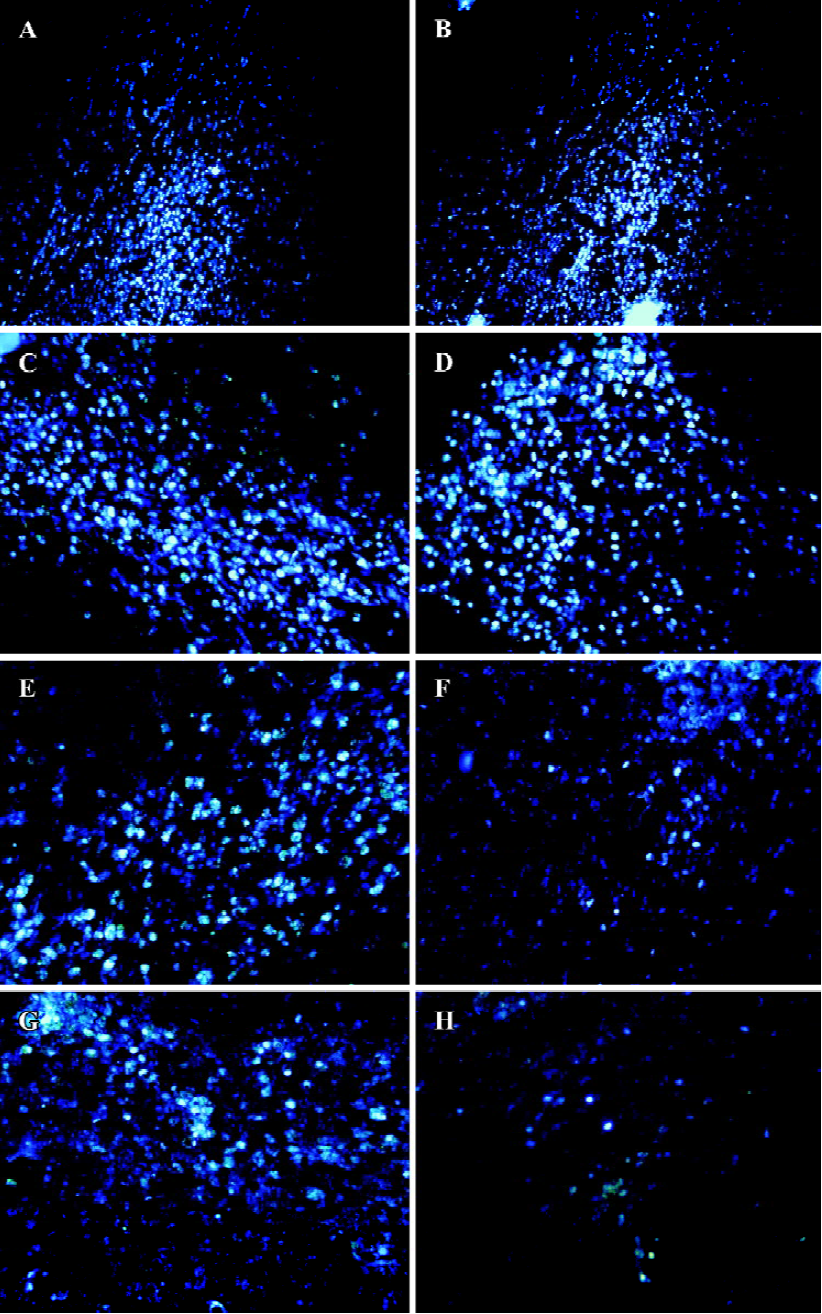
For the experiments in vivo, 3 d and 7 d after transplanta-tion, rats were anesthetized and perfused intracardially with phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde dissolved in PBS (pH 7.4). Then, serial coronal brain cryostat sections were cut at 20 µm thickness and every third section was used to count the surviving cells. To assess the survival of grafted Hoechst-stained neurons, a similar method was employed[8].
Neurons cultured with TNF-α Three days after trans-fection, TNF-α was added to A20-neurons and pcDNA3-neurons to a final concentration of 2000 kU/L, and the cells were incubated at 37 ºC in 5% CO2 for 8 h. Then, the apoptosis rates were accessed by flow cytometry using a FACStar (Becton Dickinson) with excitation wavelength set at 585 nm.
Labeling of neurons for transplantationSeventy-two hours after transfection, Hoechst 33258 (Sigma) was added to each well to achieve a final concentration of 10 mg/L. One hour later, cells were rinsed 3 times with PBS to completely flush out excess fluorescence and the cells were then collected for transplantation.
Surgical procedureAll animal experimental procedures were approved by the local animal protection authority. Twenty-four male Sprague-Dawley rats (250–300 g) were randomly allocated to four groups of equal size (group A-D). Rats in groups C and D were anesthetized with 10% chloralhydrate, a midline neck incision was made and a 30-mm long piece of 4–0 monofilament nylon suture was advanced from the external carotid artery (ECA) through the common carotid artery (CCA) and into the lumen of the internal carotid artery (ICA) until it blocked the origin of the middle cerebral artery (MCA). One and a half hours after the occlusion, the filament was withdrawn[9]. The rats were allowed to recover and were monitored daily for behavioral and neurological deficits (motor weakness of extremities). The rats that did not show neurological deficits were excluded [10].
Transplantation surgery Immediately after MCAO, the rats’ heads were immobilized in a stereotaxic frame. Either A20 or pcDNA3 neurons (3×105 cells) 2 µL were injected into the penumbra at the following coordinates: 1.00 mm rostral to bregma, 3.0 mm right of the midline, and 1.2 mm ventral from the dural surface, with the incisor bar set at zero. Another 12 normal rats serving as controls received identical grafts[11]. Rats were divided into 4 groups: normal rats with A20-neurons (group A), normal rats with pcDNA3-neurons (group b), MCAO rats with A20-neurons (group C), MCAO rats with pcDNA3-neurons (group D).
Statistical analysisData are expressed as mean±SD. Statistical analysis was performed by one-way ANOVA and the t-test, using statistical software package SPSS 10.0. Statistical significance was set at a level of P<0.05.
Results
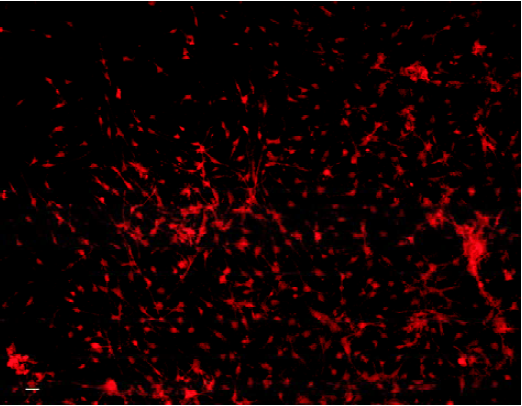
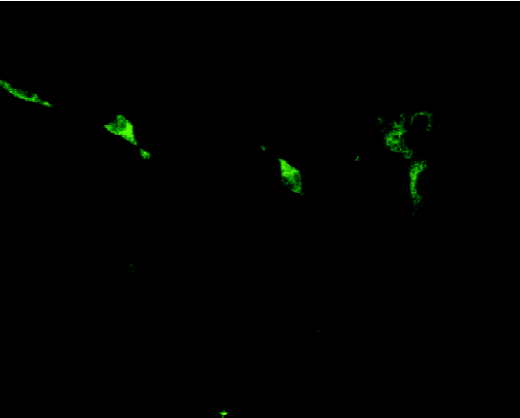
Identification of primary cultured cells and evaluation of transfection efficiency The fact that most cells were immunoreactive for NF indicates that most cells were neurons (Figure 1). Seventy-two hours after gene delivery, neurons immunoreactive for A20 were counted as described above for quantitative determination of transfection efficiency (Figure 2). The percentage of neurons successfully transfected with A20 was 52.46%±5.26%.
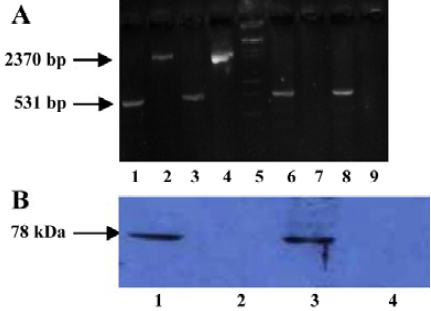
Expression of A20 in neurons in vitro and after transplantion into MCAO brainsActivation of A20 was detected 10 d after transfection by both RT-PCR (Figure 3A) and Western blot analysis (Figure 3B).
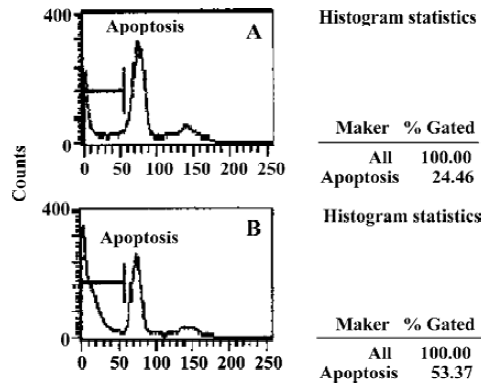
Induction of apoptosis by TNF-α in vitroThe results of FACS analysis showed that after the proapoptotic insult with TNF-α, the apoptosis rate of neurons overexpressing A20 (28.46%±3.87%) was lower than that in neurons transfected with pcDNA3 (53.06%±5.36%) (P<0.05, Figure 4A, 4B).
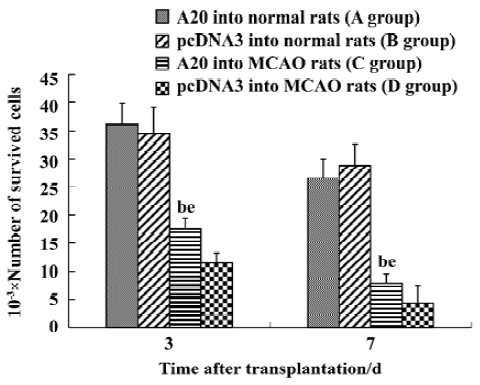
A20 protected the transplanted neurons from apo-ptosis in the penumbra of the MCAO brainCells derived from the hippocampus of E18 Sprague-Dawley rats were transfected with A20 or pcDNA3 and grafted into the apoptotic zone (penumbra) of MCAO rats, and at an identical position in normal rats. Grafts were identified in vivo 3 d and 7 d after transplantation by staining using the Hoechst 33258 stain. A large number of cells implanted into the normal rat brain survived, with no significant difference in the cell survival rate between the A20 group and the pcDNA3 group on d 3 or d 7 (P>0.05). On the other hand, a great deal of grafted cells died when transplanted into the apoptotic zone of the ischemic brain. In this case, neurons with A20 that were grafted into MCAO rats had a greater survival rate compared to pcDNA3 cells both on d 3 and d 7 (P<0.05, Figure 5A-5H, 6).
Discussion
A20 is encoded by a primary response gene which was originally identified as a TNF-inducible gene in human umbilical vein endothelial cells[12]. It seems to have a dual activities, serving not only as an inhibitor of NF-κB activation, but also as an antiapoptotic gene in some cell systems. Primary cells that are taken directly from the body are morphologically and physiologically similar to the parent tissue. Gene transfer using primary cells is a much better way to mimic the in vivo situation. This method permits much more precise medical and scientific conclusions than when genes are transferred into degenerated cell lines[14].
In our research, we used rat primary hippocampal neurons, which form an important in vitro model for the research of many central nervous system diseases (such as brain ischemia and Parkinson disease) and for studying routine events in most parts of the brain. And a newly developed electroporation method known as Nucleofector technology was employed in the study of the rat primary hippocampal neurons. It is a physical method that is suitable for the transfection of nonphagocytic and nonproliferating cells, such as peripheral leukocytes and stem cells, which are usually refractory to chemical transfection vectors. In addition, it is free of biocontaminants and does not induce immune reactions[15]. The results of immunocytochemical analysis indicated that Nucleofector technology obtained a 52.46%±5.26% transfection efficiency for the A20 gene, which is the highest transfection efficiency reported so far involving nonviral gene transfer of primary cell cultures. This high rate of efficiency is important for advanced functional experiments. RT-PCR and Western bloting analysis further proved the continuous expression of A20 proteins both in vitro and in vivo 10 d after transfection.
FACS analysis demonstrated that the A20 could protect the primary culture neurons from TNF-induced apoptosis in vitro. The mechanism of action has not yet been completely clarified. Some researchers speculate that A20 might interfere with TNF-R-associated death domain (TRADD) binding to TNF-R1 and might, thus, negatively regulate TNF induced cytotoxicity[6].
Most of the evidence supporting the role of apoptosis in neuronal cell death comes from studies using animal models of global or focal cerebral ischemia. In the focal ischemia models, which create a condition emulating human strokes, transient ischemia followed by reperfusion is often associated with massive induction of apoptosis-like cell death[16]. After reversible middle cerebral artery occlusion in adult rats, both TNF and TRAIL proteins are expressed in the apoptotic areas of the post-ischemic brain (the penumbra), thus providing conditions for inducing apoptosis. TRAIL mRNA levels increase in response to ischemia and reperfusion, reaching a maximum after 3 d. In contrast to TRAIL, TNF-α mRNA is not consistently upregulated in the ischemic hemisphere. TNF-α mRNA exhibits a first peak after 24 h, followed by a decline after 3 d, and a second rise after 5 d[17]. Furthermore, in focal cerebral ischemia, NF-κB is activated and promotes cell death[17,18]. So when cultured neurons are injected into this zone, apoptosis is expected to occur. Cell counting of surviving neurons in the present study confirmed this speculation. Higher levels of cell death were observed in the case of both A20-neurons and pcDNA3-neurons when grafted into MCAO rats than when grafted into normal rats. This result proves that apoptosis-inducing circumstances definitely exist in the penumbra following transient cerebral ischemia. On the other hand, among the two groups of MCAO rats, more A20-neurons survived than the sham group pcDNA3-neurons. This result indicates that A20 protein can protect the neurons from the cytotoxicity associated with the ischemic zone. Some further points should be made. First, the transfection efficiency was approximately 50%, and neurons that did not express the A20 protein would likely be induced to undergo apoptosis. Second, the transplanted neurons were influenced not only by TNF and TRAIL, but also by multiple other toxic factors, such as the CD95-ligand, starvation, etc. Thus 7 d after transplantation into the ischemic zone, the fact that large number of A20-neurons nevertheless died indicates that the function of A20 protein can only partly and not completely protect the neurons in vivo.
In conclusion, our study showed that the zinc finger protein A20 was an effective neuroprotective agent that improved the survival of both cultured and grafted embryonic hippocampal neurons, which suggests that the A20 may have therapeutic potential in gene therapy for diseases of the central nervous system.
Acknowledgment
We would like to thank Mr Zhi-hua JIANG, and Miss Sheng LI for their technical and material assistance.
Footnote
Project supported by Major State Basic Research Development Program of China (N
References
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;267:1456-62.
- Opipari AW Jr, Hu HM, Yabkowitz R, Dixit VM. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem 1992;267:12424-7.
- Yu LY, Lin B, Zhang ZL, Guo LH. Direct transfer of A20 gene into pancreas protected mice from streptozotocin-induced diabetes. Acta Pharmacol Sin 2004;25:721-6.
- Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci USA 1996;93:6721-5.
- Ferran C, Stroka DM, Badrichani AZ, Cooper JT. A20 inhibits NF-kappaB activation in endothelial cells without sensitizing to tumor necrosis factor-mediated apoptosis. Blood 1998;91:2249-58.
- Beyaert R, Heyninck K, Van Huffel S. A20 and A20-binding proteins as cellular inhibitors of nuclear factor-kappa B-dependent gene expression and apoptosis. Biochem Pharmacol 2000;60:1143-51.
- Costantini LC, Bakowska JC, Breakefield XO, Isacson O. Gene therapy in the CNS. Gene Therapy 2000;7:93-109.
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991;231:482-97.
- Stephenson D, Yin T, Smalstig EB, Hsu MA, Clemens J. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J Cereb Blood Flow Metab 2000;20:592-603.
- Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Schneiderman N, et al. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res 1992;575:238-46.
- Yao H, Takasawa R, Fukuda K, Shiokawa D, Sadanaga-Akiyoshi F, Ibayashi S, et al. DNA fragmentation in ischemic core and penumbra in focal cerebral ischemia in rats. Brain Res Mol Brain Res 2001;91:112-8.
- Dixit VM, Green S, Sarma V, Holzman LB, Wolf FW, O’Rourke K, et al. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem 1990;265:2973-8.
- Grossmann M, Nakamura Y, Grumont R, Gerondakis S. New insights into the roles of ReL/NF-κB transcription factors in immune function, hemopoiesis and human disease. Int J Biochem Cell Biol 1999;31:1209-19.
- Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med 2003;348:1356-64.
- Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO J 1982;1:841-5.
- Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci 1999;893:243-53.
- Martin-Villalba A, Herr I, Jeremias I, Hahne M, Debatin KM, et al. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci 1999;19:3809-17.
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 1999;5:554-9.